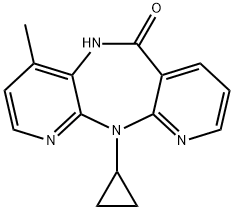
Nevirapine synthesis
- Product Name:Nevirapine
- CAS Number:129618-40-2
- Molecular formula:C15H14N4O
- Molecular Weight:266.3
133627-46-0
186 suppliers
$54.00/5g
765-30-0
483 suppliers
$10.00/25g

129618-40-2
318 suppliers
$5.00/250mg
Yield:129618-40-2 86%
Reaction Conditions:
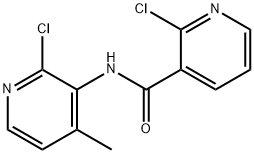
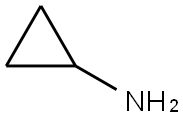
Stage #1:2-chloro-N-(2-chloro-4-methyl-3-pyridinyl)-3-pyridinecarboxamide;Cyclopropylamine with triethylamine in toluene at 130; for 10 h;Autoclave;
Stage #2: with sodium amide in diethylene glycol dimethyl ether at 15 - 110; for 2 h;Autoclave;Temperature;Time;Reagent/catalyst;
Steps:
1
200ml of toluene was added in the autoclave, 2-chloro -N- (2-chloro-4-methyl-3-pyridinyl) -3-pyridinecarboxamide (100.0g, 0.35mol), triethylamine (100ml, 0.72 mol), cyclopropylamine (51.0ml, 0.74mol), stirred open. It was slowly heated to 130 , incubated for 10 hours. Sample testing surplus stock.Opening the autoclave, the above mixture into the reaction vessel, was added diglyme 100ml, stirring, cooling to 15 , was added sodium amide (44.0g, 1.13mol), stirred for 10 minutes incubation. Heating slowly warmed to 110 , incubated for 2 hours. After the reaction monitored by TLC starting material, toluene was distilled off under reduced pressure. The residue was cooled to 20 , water was added dropwise 500ml, pale yellow solid gradually precipitated. An aqueous solution of 50% glacial acetic acid was added dropwise under ice-water bath, neutralized to pH 7-7.5, stirred for half an hour. Filtration, the filter cake washed with 50ml water, and air dry to give crude nevirapine.Added to the above crude product in dichloromethane 200ml, warmed to reflux, beating one hour, cooled to 5 , suction filtered after stirring for half an hour. The filter cake was added percentage of 55% by weight aqueous solution of 1000ml of ethanol, warmed to reflux, stirred for half an hour, the solid dissolved, active carbon, hot filtration, the filtrate was distilled at atmospheric pressure to a volume of about 500 ml, 1 hour iced water, suction to obtain purified nevirapine 80.2g, yield 86%, HPLC purity 99.8% detection.
References:
JIANGSU PUXIN PHARMACEUTICAL DEVELOPMENT.CO.LTD;Li, JinLiang;Zhao, Nan;Wu, Jun CN103183678, 2016, B Location in patent:Paragraph 0089-0091
133627-47-1
35 suppliers
$189.00/250mg

129618-40-2
318 suppliers
$5.00/250mg
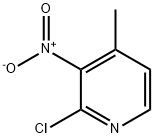
23056-39-5
327 suppliers
$6.00/1g

129618-40-2
318 suppliers
$5.00/250mg
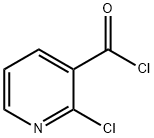
49609-84-9
231 suppliers
$20.00/5g

129618-40-2
318 suppliers
$5.00/250mg
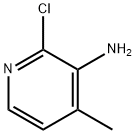
133627-45-9
484 suppliers
$9.00/1g

129618-40-2
318 suppliers
$5.00/250mg