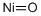
Nickel(II) oxide synthesis
- Product Name:Nickel(II) oxide
- CAS Number:1313-99-1
- Molecular formula:NiO
- Molecular Weight:74.69
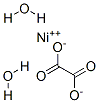
547-67-1
70 suppliers
inquiry
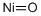
1313-99-1
314 suppliers
$20.00/100 gm
Yield:-
Reaction Conditions:
in neat (no solvent)byproducts: Ni; thermal decomposition;;
References:
Boulle, A.;David, R. [Comptes Rendus Hebdomadaires des Seances de l'Academie des Sciences,1953,vol. 243,p. 495 - 498]