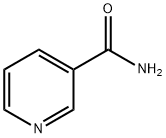
Nicotinamide synthesis
- Product Name:Nicotinamide
- CAS Number:98-92-0
- Molecular formula:C6H6N2O
- Molecular Weight:122.12
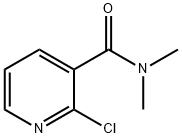
52943-21-2
52 suppliers
$40.95/250mg

98-92-0
1152 suppliers
$5.00/5g
Yield:98-92-0 96%
Reaction Conditions:
Stage #1: 2-chloropyridine-3-carboxylic acid N,N-dimethylamidewith sodium thiosulfate * 6H2O in lithium hydroxide monohydrate at 77; for 6 h;
Stage #2: with chlorine in lithium hydroxide monohydrate at 0; for 1 h;
Steps:
1.1-1.3; 2.1-2.3; 3.1-3.3 A preparation method of nicotinamide, the steps are as follows:
(1) in 500mL four-necked flask, drop into 60g 2-chloro-N,N-dimethylnicotinamide and 100mL water, be warming up to 77 , drip the sodium thiosulfate aqueous solution that 200g concentration is 45wt%, drop home After the end, the reaction was incubated at 77° C. for 6 hours, and sampling was performed for analysis. When the content of 2-chloro-N,N-dimethylnicotinamide was less than 0.3%, the temperature was lowered to prepare theAqueous solution of sodium dimethylnicotinamide sulfonate;(2) in a 1000mL four-necked flask, at 0°C, add the dimethylnicotinamide sulfonic acid sodium salt aqueous solution described in step (1) into 200mL of water, and feed chlorine gas (at 0.587g) into the water while adding. /min rate, the introduction time is 3.5 hours), then after the reaction was incubated at 0 °C for 1 h, dichloromethane was added to obtain a dichloromethane mother liquor, which wasThe dichloromethane mother liquor is used after washing with water;(3) the extraction mother liquor after the washing described in step (2) is cooled to 5 and then feeds 17g (1mol) of ammonia gas, adds 100mL of water after the completion of the ammonia gas flow, drips hydrochloric acid to adjust the pH of the system after pH=2, cools down to -5°C, then centrifuged, washed with clean water, and then vacuum-dried to obtain 96.97 g of the product with a yield of 96% and a purity of 98.5%.That is, the nicotinamide is prepared.
References:
CN114940662,2022,A Location in patent:Paragraph 0027-0041
100-55-0
391 suppliers
$8.00/5g

98-92-0
1152 suppliers
$5.00/5g
500-22-1
460 suppliers
$6.00/5g

98-92-0
1152 suppliers
$5.00/5g
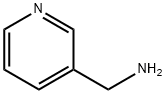
3731-52-0
330 suppliers
$5.00/5g

98-92-0
1152 suppliers
$5.00/5g
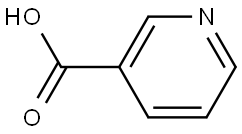
59-67-6
1084 suppliers
$7.00/25g

98-92-0
1152 suppliers
$5.00/5g