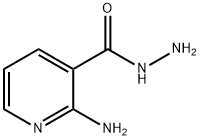
Nicotinic acid, 2-amino-, hydrazide synthesis
- Product Name:Nicotinic acid, 2-amino-, hydrazide
- CAS Number:5327-31-1
- Molecular formula:C6H8N4O
- Molecular Weight:152.15
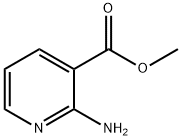
14667-47-1
202 suppliers
$6.00/1g

5327-31-1
33 suppliers
inquiry
Yield:5327-31-1 96%
Reaction Conditions:
with hydrazine hydrate in ethanol at 90; for 15 h;Inert atmosphere;
Steps:
5-Bromo-3-(5-tert-butyl-1,3,4-oxadiazol-2-yl)pyridin-2-amine (2b)
A solution of hydrazine hydrate (3.2 mL, 66 mmol) was added to methyl 2-aminonicotinate 2a (1 g, 6.57 mmol) in ethanol (25 mL). The resulting suspension was stirred at 90°C for 15 hours. The mixture was evaporated under reduced pressure, triturated twice in a mixture of 50:50 acetonitrile / dichloromethane, evaporated under reduced pressure. The resulting solid was triturated in diethyl ether, filtered, washed with diethyl ether and dried under reduced pressure to afford 2-aminonicotinohydrazide (0.960 g, 96%) as a beige solid, previously described in: Oakes, V.; Pascoe,R.; Rydon, H. N. J. Chem. Soc. 1956, 1045. EDCI (753 mg, 3.93 mmol) was added in one portion to 2-aminonicotinohydrazide (460 mg, 3.02 mmol), pivalic acid (0.35 mL, 3.0 mmol) and HOPO (437 mg, 3.93 mmol) dissolved in DMF (5 mL). The resulting solution was stirred at 25 °C for 15 hours. The reaction mixture was concentrated and adsorbed on silica gel. The crude product was purified by flash chromatography on silica gel eluting with 3 to 12% methanolic ammonia (7 N) in dichloromethane. The solvent was evaporated to dryness to afford 2-amino-N'-pivaloylnicotinohydrazide (520 mg, 73%) as a white solid. N,N’-diisopropylcarbodiimide (0.2 mL, 1.3 mmol) was added to a stirred mixture of 2-amino-N'-pivaloylnicotinohydrazide (300 mg, 1.27 mmol) in DMA (3 mL). The resulting mixture was heated at 130 °C for 4 h. The solvent was evaporated under reduced pressure and adsorbed on silica gel. The crude product was purified by flash chromatography on silica gel eluting with 1 to 3% methanol in dichloromethane. The solvent was evaporated to dryness to afford 3-(5-tert-butyl-1,3,4-oxadiazol-2-yl)pyridin-2-amine (212 mg, 76%) as a pale white crystalline solid. NBS (135 mg, 0.76 mmol) was added to a stirred solution of 3-(5-tert-butyl-1,3,4-oxadiazol-2-yl)pyridin-2-amine (150 mg, 0.69 mmol) dissolved in tetrahydrofuran (3 mL). The mixture was stirred at 25 °C for 2 h. The mixture was evaporated under reduced pressure, and the solid was tritured with water, filtered and dried with P2O5 under reduced pressure to afford 5-bromo-3-(5-tert-butyl-1,3,4-oxadiazol-2-yl)pyridin-2-amine 2b (180 mg, 88%) as a white solid.
References:
Barlaam, Bernard;Cosulich, Sabina;Delouvrié, Bénédicte;Ellston, Rebecca;Fitzek, Martina;Germain, Hervé;Green, Stephen;Hancox, Urs;Harris, Craig S.;Hudson, Kevin;Lambert-Van Der Brempt, Christine;Lebraud, Honorine;Magnien, Fran?oise;Lamorlette, Maryannick;Le Griffon, Antoine;Morgentin, Rémy;Ouvry, Gilles;Page, Ken;Pasquet, Georges;Polanska, Urszula;Ruston, Linette;Saleh, Twana;Vautier, Michel;Ward, Lara [Bioorganic and Medicinal Chemistry Letters,2015,vol. 25,# 22,p. 5155 - 5162] Location in patent:supporting information
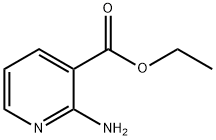
13362-26-0
203 suppliers
$7.00/1g

5327-31-1
33 suppliers
inquiry