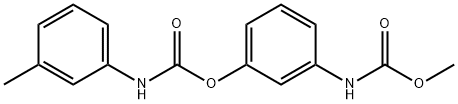
Phenmedipham synthesis
- Product Name:Phenmedipham
- CAS Number:13684-63-4
- Molecular formula:C16H16N2O4
- Molecular Weight:300.31
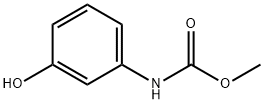
13683-89-1
26 suppliers
inquiry
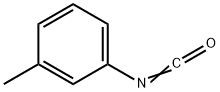
621-29-4
189 suppliers
$10.00/1g
620-50-8
3 suppliers
inquiry

13684-63-4
145 suppliers
$57.89/100mg
Yield:13684-63-4 100%
Reaction Conditions:
with borax in water
Steps:
6 Preparation of methyl (3-(3-tolyl-carbamoyloxy)phenyl)carbamate
EXAMPLE 6 Preparation of methyl (3-(3-tolyl-carbamoyloxy)phenyl)carbamate Methyl N-(3-hydroxyphenyl)carbamate (8.35 g, 0.05 mole) (prepared as described in Example 1) in water (75 ml) was placed in a 150 ml beaker equipped with a stirrer, and the mixture was stirred. Borax (2.0 g) was added, and over a period of 3/4 hour 3-tolyl isocyanate (5.3 g, 0.04 mole) was added by means of a separatory funnel without any adjustment of temperature. The pH value was 9.2 during the reaction time. After the addition of the 3-tolyl isocyanate, the stirring was continued for 1 hour. Subsequently the product, which was a very fine, suspended powder, was filtered, washed and dried to yield the title product (12.0 g, 100% calculated on the isocyanate intermediate), m.p. 139.8°-141.8° C. HPLC-Analysis showed a purity of 95.2%, the remaining 4.8% being methyl N-(3-hydroxyphenyl)carbamate and a by-product, N,N'-di-3-tolylurea.
References:
Kemisk Vaerk Koge A/S US4748265, 1988, A