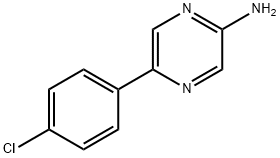
PyrazinaMine, 5-(4-chlorophenyl) synthesis
- Product Name:PyrazinaMine, 5-(4-chlorophenyl)
- CAS Number:59489-72-4
- Molecular formula:C10H8ClN3
- Molecular Weight:205.64
Yield:59489-72-4 81%
Reaction Conditions:
with sodium carbonate;tetrakis(triphenylphosphine) palladium(0) in 1,4-dioxane;methanol at 110; for 2.5 h;
Steps:
48.1a.
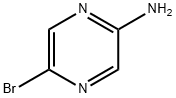
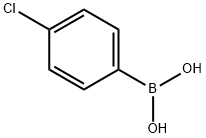
48.1a. 5-(4-chlorophenyl)pyrazin-2-ylamine 50 mL of 2N sodium carbonate solution and 1.16 g (1.00 mmol) of Pd(PPh3)4 were added to a solution of 8.70 g (174 mmol) of 5-bromopyrazin-2-ylamine and 7.98 g (156 mmol) of 4-chlorophenylboric acid in 150 mL of 1,4-dioxane and 50 mL of MeOH and the mixture was heated to 110° C. for 2.5 hours. The reaction mixture was evaporated down in vacuo and diluted with EtOAc. The organic phase was washed with water, dried over sodium sulfate, and evaporated down in vacuo. The residue was purified by column chromatography (silica gel, DCM→DCM/MeOH 20:1). Yield: 8.30 g (81% of theoretical); C10H8ClN3 (M=205.643); calc.: molpeak (M+H)+: 206/208 (Cl); found: molpeak (M+H)+: 206/208 (Cl); HPLC-MS: 7.15 minutes (method G).
References:
US2005/234101,2005,A1 Location in patent:Page/Page column 52