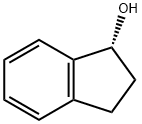
(R)-(-)-1-INDANOL synthesis
- Product Name:(R)-(-)-1-INDANOL
- CAS Number:697-64-3
- Molecular formula:C9H10O
- Molecular Weight:134.18
83-33-0
543 suppliers
$6.00/5g

697-64-3
73 suppliers
$70.00/50mg
25501-32-0
58 suppliers
$115.00/100mg
Yield: 99%
Reaction Conditions:
with dimethylsulfide borane complex;(S)-2-(anilinodiphenylmethyl)pyrrolidine in tetrahydrofuran at 20; for 2 h;enantioselective reaction;
Steps:
Catalytic enantioselective borane reduction of acetophenone by using (S)-2-(anilinodiphenylmethyl)pyrrolidine ((S)-3): typical procedure
General procedure: To a THF (1.25 mL) solution of (S)-2-(anilinodiphenylmethyl)pyrrolidine ((S)-3) (99 mg, 0.30 mmol) was added borane-dimethyl sulfide complex (0.12 mL, 1.3 mmol) at 0 °C, and the mixture was refluxed for 45 min with stirring. After the mixture was cooled to room temperature, acetophenone (0.33 mol dm-3 THF solution, 3.0 mL, 1.0 mmol) was added dropwise via syringe pump over 2 h, and stirring was continued for 2 h at room temperature. Then 1 mol dm-3 aqueous HCl was added at 0 °C, and the reaction mixture was stirred at room temperature for 30 min. The aqueous layer was separated and extracted with ether, and the combined organic layers were washed with 1 mol dm-3 aqueous HCl, water, and brine, successively. The organic layer was dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure. The resulting crude product was purified by preparative TLC (chloroform) to give (R)-1-phenylethanol (118 mg, 0.96 mmol, 96%). The ee was determined to be 94% by HPLC analysis using a chiral column (Daicel Chiralcel OD-H (25*0.46 cm i.d.)); eluent, 5% 2-propanol in hexane; flow rate, 0.5 mL/min; tR, 17.5 min for major peak, 21.0 min for minor peak.
References:
Hosoda, Naoya;Kamito, Hideaki;Takano, Miki;Takebe, Yoshitaka;Yamaguchi, Yoshitaka;Asami, Masatoshi [Tetrahedron,2013,vol. 69,# 6,p. 1739 - 1746]

83-33-0
543 suppliers
$6.00/5g

697-64-3
73 suppliers
$70.00/50mg

26452-98-2
28 suppliers
$461.00/5g

697-64-3
73 suppliers
$70.00/50mg

68567-23-7
0 suppliers
inquiry

879-35-6
0 suppliers
inquiry
6351-10-6
157 suppliers
$6.00/5g

697-64-3
73 suppliers
$70.00/50mg

25501-32-0
58 suppliers
$115.00/100mg

83-33-0
543 suppliers
$6.00/5g

6351-10-6
157 suppliers
$6.00/5g

697-64-3
73 suppliers
$70.00/50mg