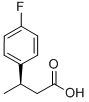
(R)-3-(4-FLUOROPHENYL)BUTANOIC ACID synthesis
- Product Name:(R)-3-(4-FLUOROPHENYL)BUTANOIC ACID
- CAS Number:209679-21-0
- Molecular formula:C10H11FO2
- Molecular Weight:182.19
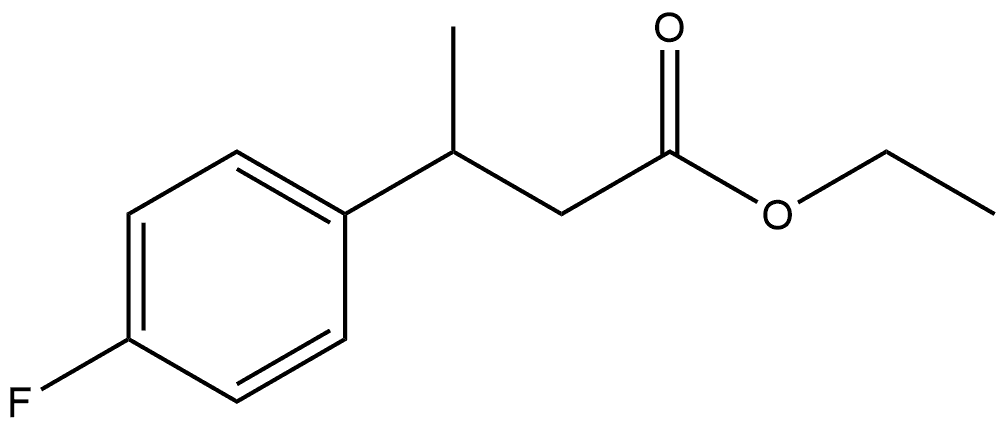
162549-00-0
1 suppliers
inquiry

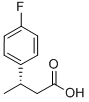
209679-21-0
11 suppliers
inquiry
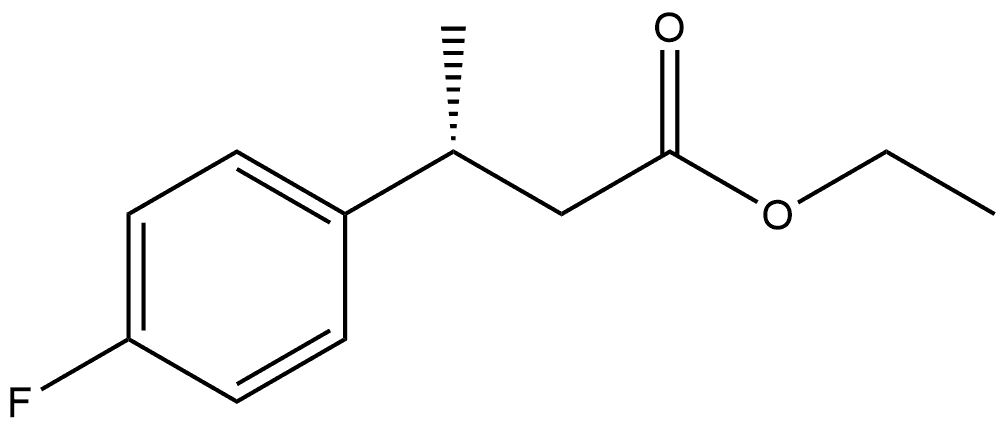
209679-20-9
10 suppliers
inquiry
1286204-36-1
1 suppliers
inquiry
Yield:-
Reaction Conditions:
with Pseudomonas cepacia P1 hydrolase;water at 30; pH=7; for 64 h;aq. phosphate buffer;Enzymatic reaction;optical yield given as %ee;enantioselective reaction;
Steps:
4.11.1. General procedure for the hydrolase catalysed kinetic resolution of the 3-aryl alkanoic ethyl esters (+/-)-3a-i (analytical scale)
General procedure: A spatula tip of enzyme (5-10 mg) was added to the substrate (+/-)-3a-i (50 mg) in 0.1 M phosphate buffer, pH 7 (4.5 mL). Co-solvents (17% v/v) were added as indicated in Table 3. The reaction vessel was shaken at 700-750 rpm and incubated at the appropriate temperature for the required length of time. The aqueous layer was extracted with diethyl ether (3 × 5 mL) and the combined organic extracts were filtered through Celite and concentrated under reduced pressure. The sample was analysed by 1H NMR spectroscopy, reconcentrated and dissolved in a mixture of hexane/iso-propyl alcohol (HPLC grade) and enantioselectivity determined by chiral HPLC. The results of the screen are summarised in the appropriate [Table 1] and [Table 10].
References:
Deasy, Rebecca E.;Brossat, Maude;Moody, Thomas S.;Maguire, Anita R. [Tetrahedron Asymmetry,2011,vol. 22,# 1,p. 47 - 61] Location in patent:experimental part
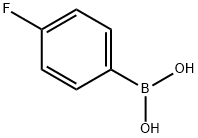
1765-93-1
547 suppliers
$9.00/1g

209679-21-0
11 suppliers
inquiry

115621-54-0
0 suppliers
inquiry

209679-21-0
11 suppliers
inquiry

209679-20-9
10 suppliers
inquiry

1286204-36-1
1 suppliers
inquiry
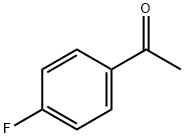
403-42-9
533 suppliers
$5.00/10g

209679-21-0
11 suppliers
inquiry

209679-20-9
10 suppliers
inquiry

1286204-36-1
1 suppliers
inquiry
