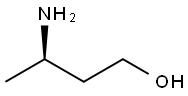
(R)-3-amino-1-butanol synthesis
- Product Name:(R)-3-amino-1-butanol
- CAS Number:61477-40-5
- Molecular formula:C4H11NO
- Molecular Weight:89.14

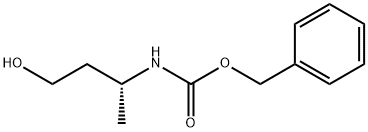
866395-21-3
8 suppliers
inquiry

61477-40-5
426 suppliers
$15.00/1g
Yield:61477-40-5 96%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in ethanol at 50 - 60; under 3000.3 - 4500.45 Torr;
Steps:
4 Example 4: Synthesis of (R) -3-aminobutanol
Put 50g (R) -3-benzyloxyamidobutanol in a clean four-necked bottle, add 200ML ethanol, 2.5g10wt% Pd / C, heat up to 50 60 , hydrogen pressure 0.4 0.6MPa, react until the raw materials disappear . After filtration, the filtrate was concentrated under reduced pressure to obtain an oily substance (R) -3-aminobutanol. Yield: 96.0%, purity 99.7%, ee: 99.9%.
References:
CN110683960,2020,A Location in patent:Paragraph 0051; 0052
1236049-43-6
2 suppliers
inquiry

61477-40-5
426 suppliers
$15.00/1g
3775-73-3
275 suppliers
$8.00/1g

61477-40-5
426 suppliers
$15.00/1g
![Carbamic acid, [(1R)-3-hydroxy-1-methylpropyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/GIF/167216-17-3.gif)
167216-17-3
43 suppliers
inquiry

61477-40-5
426 suppliers
$15.00/1g
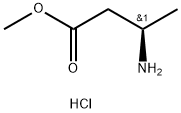
139243-54-2
68 suppliers
$9.00/1g

61477-40-5
426 suppliers
$15.00/1g