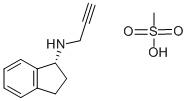
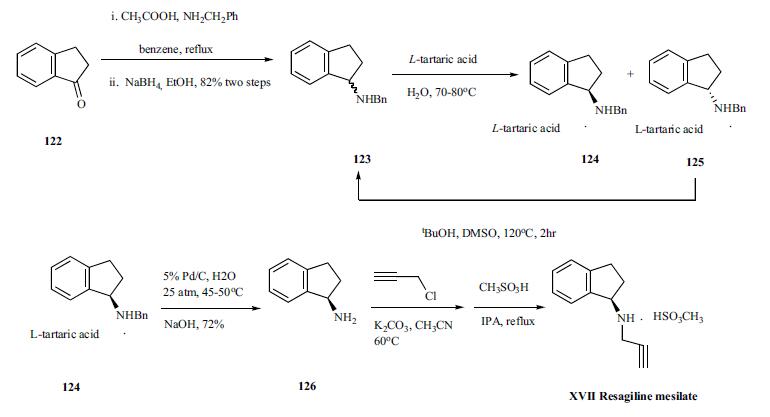
Rasagiline mesylate synthesis
- Product Name:Rasagiline mesylate
- CAS Number:161735-79-1
- Molecular formula:C13H17NO3S
- Molecular Weight:267.344
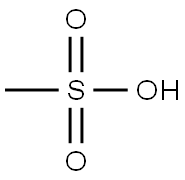
75-75-2
596 suppliers
$10.00/5g
136236-51-6
154 suppliers
$5.00/1mg

161735-79-1
363 suppliers
$8.00/50mg
Yield:161735-79-1 98%
Reaction Conditions:
in isopropyl alcohol; for 1 h;Reflux;
Steps:
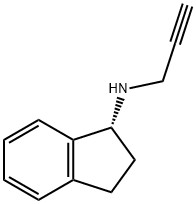
2.10. Synthesis of (R)-rasagiline mesylate (R-7)
A suspension of (R)-N-propargyl-1-aminoindan (R-6) (50 mg,0.29 mmol) in 5 mL of isopropanol and 3 L of methanesulfonic acidwas heated to reflux for 1 h. After, the mixture was allowed to coolto 5C. The obtained suspension was filtered, and the collected solidwas washed with 1.5 mL of isopropanol, affording the correspond-ing optically active (R)-rasagiline mesylate (R-7) as a white solid in98% yield.
References:
De Mattos, Marcos Carlos;De Fonseca, Thiago Sousa;Da Silva, Marcos Reinaldo;De Oliveira, Maria Da Concei??o Ferreira;De Lemos, Telma Leda Gomes;De Marques, Ricardo Araújo [Applied Catalysis A: General,2015,vol. 492,# 1,p. 76 - 82]

75-75-2
596 suppliers
$10.00/5g
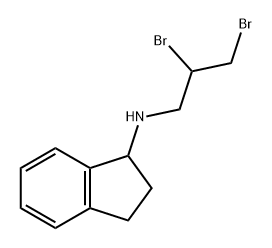
1166392-46-6
0 suppliers
inquiry

161735-79-1
363 suppliers
$8.00/50mg

75-75-2
596 suppliers
$10.00/5g
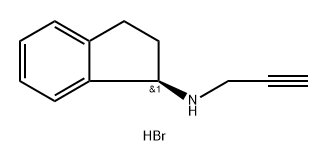
694436-33-4
0 suppliers
inquiry

161735-79-1
363 suppliers
$8.00/50mg
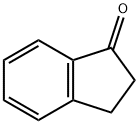
83-33-0
540 suppliers
$6.00/5g

161735-79-1
363 suppliers
$8.00/50mg