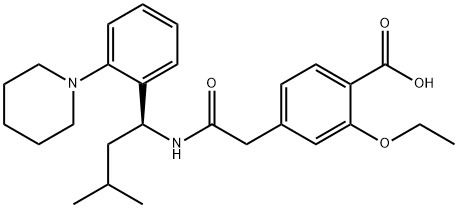
Repaglinide synthesis
- Product Name:Repaglinide
- CAS Number:135062-02-1
- Molecular formula:C27H36N2O4
- Molecular Weight:452.59
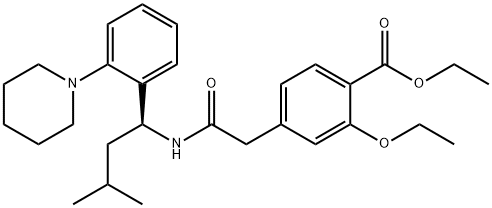
147770-06-7
101 suppliers
$50.00/5 mg

135062-02-1
561 suppliers
$5.00/10mg
Yield:135062-02-1 96%
Reaction Conditions:
with ethanol;water;sodium hydroxideReflux;
Steps:
1.3; 2.3 The third step: hydrolysis
Add 365 g of 95% ethanol, 31.9 g of Regal's ester and 30 g of 10% sodium hydroxide aqueous solution to a 1000 ml reaction kettle in sequence, and react at reflux at elevated temperature for 3 to 4 hours; sampling reaction is controlled in the middle;After the reaction is completed, the temperature is lowered to 15-20°C, hydrochloric acid is added dropwise to adjust the pH=4.0-6.0, and the temperature is kept for 1 hour; filtered, the filtrate is barreled for ethanol recovery; the filter cake is washed with an appropriate amount of purified water and then enters the refining process.Refining: Add 65g 95% ethanol, 65g purified water, and repaglinide crude wet product to a 500ml reactor in sequence, and heat to reflux; heat filtration through a positive pressure filter, and the filtrate enters the clean area.Crystal kettle; the filtrate was cooled to 10-15°C and kept warm and stirred for 1 hour; filtered and dried to obtain 113 g of white crystalline powder repaglinide, with a molar yield of 96% and an HPLC purity of 100%.
References:
Jiangxi Learned Xinhe Pharmaceutical Co., Ltd.;Wang Jian;Zhu Xianghong;Xie Xiping;Huang Lu;Ye Gang;Liu Yi CN111635379, 2020, A Location in patent:Paragraph 0083-0086; 0094-0097
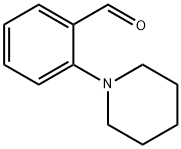
34595-26-1
82 suppliers
$11.00/500mg

135062-02-1
561 suppliers
$5.00/10mg
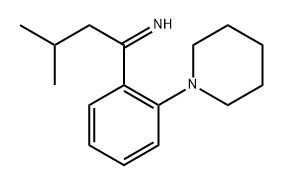
147769-96-8
3 suppliers
inquiry

135062-02-1
561 suppliers
$5.00/10mg
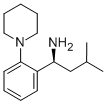
147769-93-5
192 suppliers
$34.00/250mg

135062-02-1
561 suppliers
$5.00/10mg
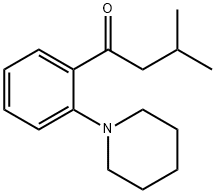
147770-03-4
7 suppliers
inquiry

135062-02-1
561 suppliers
$5.00/10mg