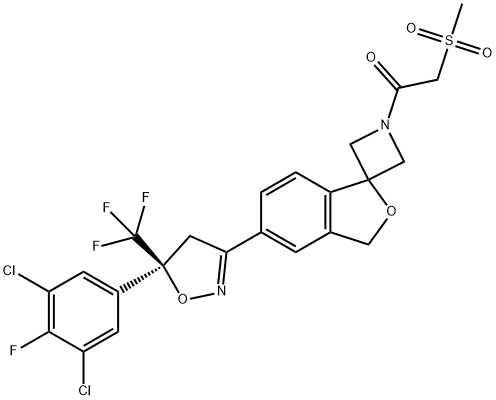
Sarolaner synthesis
- Product Name:Sarolaner
- CAS Number:1398609-39-6
- Molecular formula:C23H18Cl2F4N2O5S
- Molecular Weight:581.36
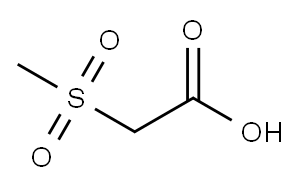
2516-97-4
132 suppliers
$8.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1398609-39-6
67 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1:2-(methylsulfonyl)acetic acid;5'-(5-(3,5-dichloro-4-fluorophenyl)-5-(trifluoromethyl)-4,5-dihydroisoxazol-3-yl)-3'H-spiro[azetidine-3,1'-isobenzofuran] besylate with triethylamine in ethyl acetate at 18 - 22; for 0.5 h;
Stage #2: with 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide in ethyl acetate at 10 - 35;
Steps:
1 Example 1 : 1 -(5'-(5-(3,5-dichloro-4-fluorophenyl)-5-(trifluoromethyl)-4,5- dihydroisoxazol-3-yl)-3'H-spiro[azetidine-3,1 '-isobenzofuran]-1 -yl)-2- (methylsulfonyl)ethanone.
Alternatively, the racemate of the besylate salt of the sulfonamide can be removed by mixing methanesulfonylacetic acid (0.615g, 1 .3eq) with the sulfonamide (2.1 g) in 9.3 mL ethyl acetate (EtOAc). Triethylamine is added dropwise over 1 minute (0.825g, 2.4eq) at about 18-22°C. The addition funnel is rinsed with 0.5 mL EtOAc and the resulting mixture is stirred for 30 minutes and cooled to <10°C. To this mixture, 4.313g n-propylphosphonic anhydride (50 weight% in EtOAc), 2.0eq) is added dropwise over 15 minutes at <10°C. The addition funnel is rinsed again with 1 .5 mL EtOAc. The reaction mixture is warmed to 35°C and stirred overnight. (UPLC >97% with <1 % starting material). To the reaction was added 1 .0g Celite filter aid (50% loading) and filtered through a 1 g celite plug in a 15 mL coarse frit glass funnel (1 .75 minute filtration) and rinsed with 4 mL EtOAc (2x). Chiral HPLC 98.8% S enantiomer, 1 .2% R; UPLC >97%, filtrate volume = 18 mL).
References:
ZOETIS LLC;WENDT, John, Adam;ASPER, David, Jose WO2014/39422, 2014, A1 Location in patent:Page/Page column 35
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1398609-39-6
67 suppliers
inquiry
![Ethanone, 1-[5'-[5-(3,5-dichloro-4-fluorophenyl)-4,5-dihydro-5-(trifluoromethyl)-3-isoxazolyl]spiro[azetidine-3,1'(3'H)-isobenzofuran]-1-yl]-2-(methylsulfonyl)-](/CAS/20210305/GIF/1398609-86-3.gif)
![Ethanone, 1-[5'-[(5R)-5-(3,5-dichloro-4-fluorophenyl)-4,5-dihydro-5-(trifluoromethyl)-3-isoxazolyl]spiro[azetidine-3,1'(3'H)-isobenzofuran]-1-yl]-2-(methylsulfonyl)-](/CAS/20210305/GIF/1398609-00-1.gif)