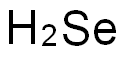
Selenium synthesis
- Product Name:Selenium
- CAS Number:7782-49-2
- Molecular formula:Se
- Molecular Weight:78.96
Because selenium is present in fossil fuels, up to 90% of the selenium content in ambient air is emitted during their combustion. Air pollution concentrations averaged from 0.38 ng/m3 in remote areas to 13 ng/m3 in urban areas. The mass medium particle diameter was 0.92 mm. The worldwide emissions of 10,000 tons/year from natural sources exceed the atmospheric emissions from anthropogenic sources (5100 ton). However, 41,000 tons is emitted into the aquatic ecosystems. The largest contributors are electric power generating plants that produce 18,000 ton; manufacturing processes account for 12,000 ton.
Most of the world’s selenium today is provided by recovery from anode muds of electrolytic copper refineries. Selenium is recovered by roasting these muds with soda or sulfuric acid or by melting them with a soda and niter.
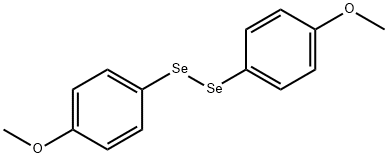
22216-66-6
0 suppliers
inquiry
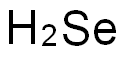
7782-49-2
233 suppliers
$27.00/25g
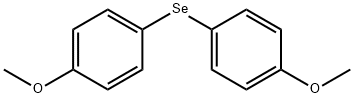
38762-70-8
1 suppliers
inquiry
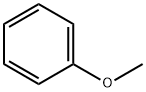
100-66-3
508 suppliers
$10.00/5G
Yield:100-66-3 41.3 %Chromat. ,38762-70-8 34 %Chromat. ,7782-49-2 22%
Reaction Conditions:
with hydrogen iodide in chloroform;water at 60; for 6 h;
Steps:
Acidolysis of compound (2).
To a solution of 0.29 g (1 mmol) of compound 2 in 5 mL of CHCl3 was added at stirring 3 mL of HI (54% water solution), themixture was heated at 60°C and kept for 6 h. Chloroform solution was separated from water layer and filtered. We isolated 17 mg (22%) of selenium. According to GC-MS data the chloroform solutioncontained anisole (41.3%), initial compound 2(21.4%), and bis(4-methoxyphenyl) diselenide (34%).
References:
Balina;Russkikh;Orlova;Ogneva;Shelkovnikov [Russian Journal of Organic Chemistry,2015,vol. 51,# 2,p. 198 - 201][Zh. Org. Khim.,2015,vol. 51,# 2,p. 210 - 213,4]
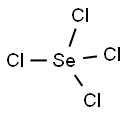
10026-03-6
55 suppliers
$55.00/10g
7782-49-2
233 suppliers
$27.00/25g
10025-68-0
41 suppliers
$107.00/10g
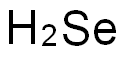
7782-49-2
233 suppliers
$27.00/25g
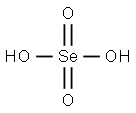
7783-08-6
70 suppliers
$79.50/25ml
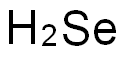
7782-49-2
233 suppliers
$27.00/25g
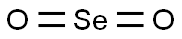
7446-08-4
265 suppliers
$13.00/5g
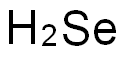
7782-49-2
233 suppliers
$27.00/25g