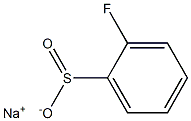
sodium:2-fluorobenzenesulfinate synthesis
- Product Name:sodium:2-fluorobenzenesulfinate
- CAS Number:127159-66-4
- Molecular formula:C6H4FNaO2S
- Molecular Weight:182.1479
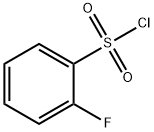
2905-21-7
221 suppliers
$9.00/5g

127159-66-4
5 suppliers
inquiry
Yield:127159-66-4 88%
Reaction Conditions:
with sodium carbonate;sodium sulfite in water at 50; for 2 h;
Steps:
SODIUM SULFINATES: EXAMPLE PROCEDURE AND DATA
General procedure: A solution of sodium sulfite (17.93g, 142.26 mmol) in H2O (100 ml) was stirred at room temperature for10 min. Sodium carbonate (23.90 g, 284.52 mmol)was added to the stirred solution.The resulting solution was stirred at 50 °C for 10 minutes.cyclopropanesulfonyl chloride (20 g, 142.26 mmol) was added dropwise to thesolution and was stirred at 50 °C for 2 hours. Thereaction mixture was evaporated to dryness and redissolved in EtOH (200 ml). The suspension was allowed to stir at roomtemperature for 20 min. The suspension was filtered and the filtrate evaporatedto afford a white solid, this was stirred with MeCN (50 ml) and thenfiltered to afford sodium cyclopropanesulfinicacid (13.70 g, 75 %) as a white solid. 1H NMR (400.132 MHz, DMSO d6)δ 1.38-1.31 (m, 1H), 0.27-0.24 (m,2H) and 0.08-0.03 (m, 2H).
References:
Blades, Kevin;Demeritt, Julie;Fillery, Shaun;Foote, Kevin M.;Greenwood, Ryan;Gregson, Clare;Hassall, Lorraine A.;McGuire, Thomas M.;Pike, Kurt G.;Williams, Emma [Tetrahedron Letters,2014,vol. 55,# 29,p. 3851 - 3855] Location in patent:supporting information