
Thieno[3,2-e]benzothiazol-2-amine, 4,5-dihydro- synthesis
- Product Name:Thieno[3,2-e]benzothiazol-2-amine, 4,5-dihydro-
- CAS Number:376349-95-0
- Molecular formula:C9H8N2S2
- Molecular Weight:208.3
Yield:-
Reaction Conditions:
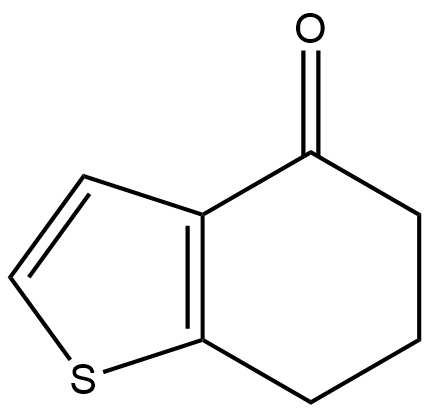
Stage #1: 4-keto-4,5,6,7-tetrahydrothianaphthene;thiourea in ethanol; for 8 h;Reflux;
Stage #2: with sodium hydroxide
Steps:
GeneralMethod II. Alternative Preparation of Benzothiazoles.
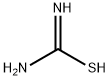
General procedure: Thiourea (17 mmol) was added to a solution of substituted ketones(6 mmol) in 30 ml of absolute ethanol. The mixture was refluxed for8 hours,which resulted in 2-aminothiazole hydrobromide salts. The free2-aminothiazole was obtained by treating the hydrobromide salt with 2MNaOH (5 ml) and extracting with ethyl acetate. The crude residue wasconcentrated, reconstituted in a methanol-water mixture (99:1), treatedwith charcoal, and recrystallized. The resulting 2-aminothiazole,2-iodoxybenzoic acid and tetrabutylammonium tribromide were combinedin ethyl acetate and stirred at roomtemperature (RT) for 10 hours.The reactionmixture was filtered through a pad of Celite, and the filtratewas diluted with saturated Na2S2O3 and extracted with ethyl acetate.The combined organic layers were dried with anhydrous sodium sulfate(anhydrous Na2SO4), concentrated, and then purified via flash chromatography(cyclohexane-EtOAc, 1:1).
References:
Coleman, Nichole;Brown, Brandon M.;Oliván-Viguera, Aida;Singh, Vikrant;Olmstead, Marilyn M.;Valero, Marta Sofia;K?hler, Ralf;Wulff, Heike [Molecular Pharmacology,2014,vol. 86,# 3,p. 342 - 357]
![5-broMo-6,7-dihydrobenzo[b]thiophen-4(5H)-one](/CAS/GIF/2513-49-7.gif)