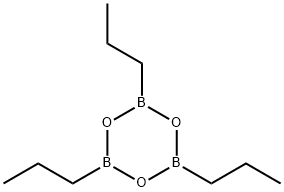
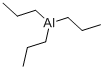
Tri-N-Propylboron synthesis
- Product Name:Tri-N-Propylboron
- CAS Number:1116-61-6
- Molecular formula:C9H21B
- Molecular Weight:140.07
Yield:1116-61-6 97.1%
Reaction Conditions:
at 100 - 140; for 5 h;
Steps:
5
Example-5 In a 1-L four-neck flask equipped with an agitator, 4 moles of tripropylaluminum was placed, and 1 mole of tripropylboroxine contained in a dropping funnel was dropped into the flask over a period of 2 hours to react with tripropylaluminum. In this case, the reaction solution was cooled with a cooling medium so as for the temperature of the reaction solution to be maintained at 100°C. Then, the flask was raised in temperature to 140°C in an oil bath, the reaction solution was aged for 3 hours, and thereafter 2.9 moles of tripropylborane at 75°C was obtained from the top of a tower packed with Dixon packing under a reduced pressure distillation condition of 36 mmHg. An aluminum oxide-containing slurry solution was left in the flask. The amounts of the aluminum oxide and the tripropylaluminum contained in the slurry solution were 0.96 mole and 2.07 moles, respectively. The yield and the purity of tripropylborane were 97.1 % and 98.5% or more, respectively.
References:
EP1903047,2008,A1 Location in patent:Page/Page column 6
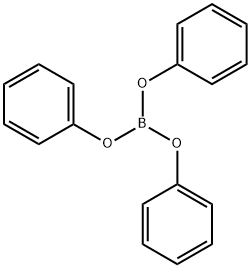
1095-03-0
131 suppliers
$20.37/5g
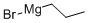
927-77-5
106 suppliers
$203.00/250g

1116-61-6
11 suppliers
inquiry
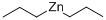
628-91-1
4 suppliers
inquiry

1116-61-6
11 suppliers
inquiry
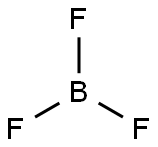
7637-07-2
5 suppliers
$35.29/10ML
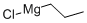
2234-82-4
100 suppliers
$63.95/100ml

1116-61-6
11 suppliers
inquiry