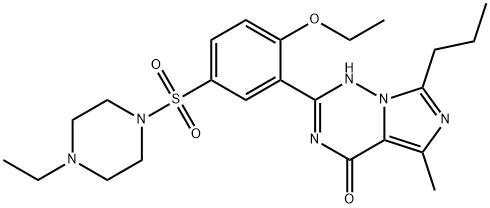
Vardenafil synthesis
- Product Name:Vardenafil
- CAS Number:224785-90-4
- Molecular formula:C23H32N6O4S
- Molecular Weight:488.6
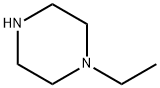
5308-25-8
444 suppliers
$15.00/5G
![2-(2-ETHOXYPHENYL)-5-METHYL-7-PROPYL-3H-IMIDAZOL[5,1-F][1,2,4]-TRIAZIN-4-ONE](/CAS/GIF/224789-21-3.gif)
224789-21-3
150 suppliers
$49.00/1g

224785-90-4
303 suppliers
$98.00/10mg
Yield:-
Reaction Conditions:
Stage #1: 2-(2-ethoxy-phenyl)-5-methyl-7-propyl-3H-imidazo[5,1-f][1,2,4]triazin-4-onewith chlorosulfonic acid at 0 - 22; for 0.75 h;
Stage #2: 4-ethylpiperazine in dichloromethane at -3 - 25; for 0.75 h;Product distribution / selectivity;
Steps:
3
EXAMPLE 3 Preparation of the Crystalline form II of Vardenafil Free Base 28 ml of chlorosulphonic acid was charged into a round bottom flask and cooled to about 0-5° C. 14 g of 2-(2-ethoxyphenyl)-5-methyl-7-propyl-3H-imidazo[5,1-f][1,2,4]-triazin-4-one was added to it at about 2° C. The temperature was raised to about 22° C. and maintained for about 45 minutes. The reaction mixture was slowly quenched into ice. 350 ml of dichloromethane was added and stirred for 25 minutes and then 150 ml of water was added and stirred for 10 minutes and the organic and aqueous layers were separated. The organic layer was charged into a round bottom flask and cooled to -3° C. 10.4 ml of N-ethylpiperazine diluted with 15 ml of dichloromethane was added at about -3° C. The temperature was raised to about 25° C. and maintained for 45 minutes. The reaction mixture was washed with 140 ml of water in two equal lots. The organic layer was completely distilled off at 41° C. and a vacuum of 300 mm Hg and then 98 ml of 4% aqueous acetone was added to it. The suspension was heated to 55° C. for dissolution. The solution was then cooled to about 2° C. and maintained for about 60 minutes for crystallization. The solid was filtered under vacuum and washed with 14 ml of pre-cooled 4% aqueous acetone solution. The solid was dried at 48° C. for about 3 hours to afford 11.8 g of the desired crystalline Form II of vardenafil free base.
References:
US2007/197535,2007,A1 Location in patent:Page/Page column 6

5308-25-8
444 suppliers
$15.00/5G
![4-Ethoxy-3-(5-methyl-4-oxo-7-propyl-3,4-dihydro-imidazo[5,1-f][1,2,4]-triazin-2-yl)benzene-sulfonyl Chloride](/CAS/GIF/224789-26-8.gif)
224789-26-8
27 suppliers
$269.85/100MG

224785-90-4
303 suppliers
$98.00/10mg
![2-(2-ETHOXYPHENYL)-5-METHYL-7-PROPYL-3H-IMIDAZOL[5,1-F][1,2,4]-TRIAZIN-4-ONE](/CAS/GIF/224789-21-3.gif)
224789-21-3
150 suppliers
$49.00/1g

224785-90-4
303 suppliers
$98.00/10mg