Applications of Octamethylcyclotetrasiloxane
Nov 13,2019
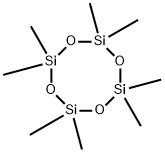
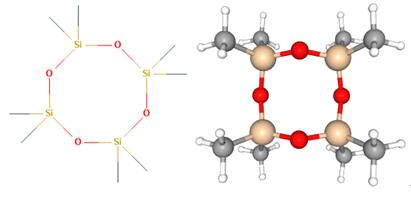
Octamethylcyclotetrasiloxane, also called D4, is an organosilicon compound with the formula [(CH3)2SiO]4. It is a colorless viscous liquid. Octamethylcyclotetrasiloxane is a common cyclomethicone. Octamethylcyclotetrasiloxane is widely used in cosmetics[1].
Commercially Octamethylcyclotetrasiloxane is produced from dimethyldichlorosilane. Hydrolysis of the dichloride produces a mixture of cyclic dimethylsiloxanes and polydimethylsiloxane. From this mixture, the cyclic siloxanes including Octamethylcyclotetrasiloxane can be removed by distillation. In the presence of a strong base such as KOH, the polymer/ring mixture is equilibrated, allowing complete conversion to the more volatile cyclic siloxanes: n/4 [(CH3)2SiO]n → n [(CH3)2SiO]4
Octamethylcyclotetrasiloxane and decamethylcyclopentasiloxane(D5) are also precursors to the polymer. The catalyst is again KOH.
Octamethylcyclotetrasiloxane is among the most important of all the cyclic siloxanes, with a global production volume of 136•million kilograms in 1993. 100,000–1,000,000 tonnes per year of Octamethylcyclotetrasiloxane is manufactured and/or imported in the European Economic Area.
Octamethylcyclotetrasiloxane is of low acute toxicity. The LC50 for a single four hour inhalation exposure in rats is 36 mg/L. The oral LD50 in rats is above 4800 mg/kg and the dermal LD50 in rats is above 2400 mg/kg[2].
Octamethylcyclotetrasiloxane and decamethylcyclopentasiloxane are cyclic siloxanes used as chemical intermediates with some applications in consumer products. The in vitro percutaneous absorption of (14)C-Octamethylcyclotetrasiloxane and (14)C-decamethylcyclopentasiloxane was studied in flow-through diffusion cells.
Single doses were applied neat and in antiperspirant formulations to dermatomed human skin for 24 hr. The majority of applied Octamethylcyclotetrasiloxane and decamethylcyclopentasiloxane (approximately 90%) volatilized before being absorbed. Only 0.5% of applied Octamethylcyclotetrasiloxane was absorbed while the absorption of decamethylcyclopentasiloxane (0.04%) was one order of magnitude lower. The largest percentage (>90%) of the absorbed Octamethylcyclotetrasiloxane and decamethylcyclopentasiloxane was found in the skin. The fate of Octamethylcyclotetrasiloxane and decamethylcyclopentasiloxane absorbed in the skin was studied in rat in vivo. A single dose of (14)C-Octamethylcyclotetrasiloxane (10, 4.8 and 2 mg/sq cm) and (14)C-decamethylcyclopentasiloxane (10 mg/sq cm) was topically applied inside a dosing chamber attached to the dorsal area. Rats were housed in metabolism cages up to 24 hr to enable collection of urine, feces, expired/escaped volatiles. The majority of applied Octamethylcyclotetrasiloxane or decamethylcyclopentasiloxane had volatilized from the skin surface. Less than 1.0% of the applied Octamethylcyclotetrasiloxane and only 0.2% of applied decamethylcyclopentasiloxane was absorbed with approximately 60% of absorbed Octamethylcyclotetrasiloxane and 30% of absorbed decamethylcyclopentasiloxane reaching systemic compartments. The amount absorbed into the skin decreased with time showing that residual Octamethylcyclotetrasiloxane and decamethylcyclopentasiloxane diffused back to the skin surface and continued to evaporate. Overall, a low tendency to pass through the skin into systemic compartments was demonstrated for both Octamethylcyclotetrasiloxane (< or = 0.5% of applied dose) and decamethylcyclopentasiloxane (<0.1% of applied dose).
As the smallest cyclic dimethylsiloxane that does not experience considerable ring strain, Octamethylcyclotetrasiloxane is one of the most abundant siloxanes in the environment, e.g. in landfill gases. decamethylcyclopentasiloxane and Octamethylcyclotetrasiloxane have attracted attention because they are pervasive. Cyclic siloxanes can be detected in some species of aquatic life.[11] An independent, peer-reviewed study in the US found "negligible risk from Octamethylcyclotetrasiloxane to organisms" while a scientific assessment by the Australian government stated, "the direct risks to aquatic life from exposure to these chemicals at expected surface water concentrations are not likely to be significant." In the European Union, Octamethylcyclotetrasiloxane was characterized as a substance of very high concern (SVHC) due to its PBT and vPvB properties and was thus included in the candidate list for authorisation.
References
[1] Moretto H, Schulze M, Wagner G. Silicones[M]// Ullmann's Encyclopedia of Industrial Chemistry. 2000.
[2] Jörg Fabri, Ulrich Graeser, Thomas A. Simo. Xylenes[M]// Ullmann's Encyclopedia of Industrial Chemistry. American Cancer Society, 2000.
- Related articles
- Related Qustion
Methyl salicylate is an external analgesic available in over-the-counter (OTC) medicines that temporarily relieve minor body aches and muscle and joint pain associated with backache, arthritis, strains, sprains, and bruises.....
Nov 13,2019Flavors and fragrancesChloroacetyl chloride, a colorless to yellow liquid, is a bi-functional compound that is useful as a chemical building block.....
Nov 13,2019Chemical ReagentsOctamethylcyclotetrasiloxane
556-67-2You may like
Octamethylcyclotetrasiloxane manufacturers
- Octamethylcyclotetrasiloxane
-

- 2025-08-11
- CAS:556-67-2
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Octamethylcyclotetrasiloxane
-

- $0.00 / 25KG
- 2025-08-08
- CAS:556-67-2
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month
- Octamethylcyclotetrasiloxane
-

- $3400.00 / 10Tons
- 2025-08-06
- CAS:556-67-2
- Min. Order: 10Tons
- Purity: 99%
- Supply Ability: 100Tons