Chloromethyl Butyrate - Reaction / Application on synthetic works
Nov 7,2019
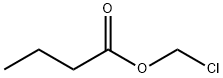
The synonyms of chloromethyl butyrate are chloromethyl n-butyrate, chloromethyl n-butanoate, n-butyric acid chloromethyl ester, propylcarbonyloxymethyl chloride, butyric acid chloromethyl ester, n-butanoyloxymethyl chloride, butyryloxymethyl chloride
Chloromethyl butyrate is an important organic intermediate to synthetize substituted esters.
Example 1
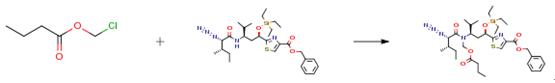
746 mg (1.27 mmole) of TES protected dipeptide benzyl ester 110 was dissolved in 8 mL of THF (anhydrous, inhibitor-free) and cooled to - 45 ° C. After 15 minutes of cooling, 2.8 mL of 0.5 M KHMDS (1.1 eq., 1.4 mmole) in toluene solution was added dropwise. After an additional 15 mins, 175 μl of chloromethyl butyrate (1.1 eq., 1.4 mmole) was added dropwise. After 30 mins, TLC showed only a trace amount of starting material left. After 2 hours, the reaction mixture was quenched 1 mL MeOH, and allowed to warm to room temperature. The reaction was extracted with EtOAc/brine. The organic layer was washed with brine and then concentrated to give 759 mg (87 percent) of the crude product.
Example 2

The 25mg alprostadil and 21μl of triethylamine were added to 10ml of ethanol, the ice bath was stirred for half an hour chloro butyrate with an appropriate amount of ethanol was dissolved 21 l of methyl chloride, was slowly added dropwise to the reaction solution, and stirring was continued at room temperature the reaction conditions 10h. It was added to the reaction solution, 50 ml ethyl acetate, and the organic phase was transferred to a separatory funnel, washed with a concentration of 3 percent aqueous citric acid until slightly acidic, then with saturated aqueous sodium bicarbonate solution once, brine to neutral. The organic layer was dried over anhydrous sodium sulfate, filtered and concentrated to give a yellow oily liquid. The raw material of alprostadil yield was 89.5 percent.
Example 3

To a solution of tegafur (0.26 gram, 1.31 mmol) in DMF (2 ml) was added K2CO3 (0.18 gram, 1.31 mmol) and the mixture was stirred for a few minutes. Chloromethylbutyrate (0.16 ml, 1.31 mmol, 1 equivalent) in 1 ml of DMF was added over 15 minutes. The reaction mixture was stirred for 20 hours at room temperature and was then filtered. To the filtrate were added toluene, water and brine. The organic layer was extracted with 5 x 15 ml of brine and the aqueous layer was extracted with 5 x 15 ml of toluene. The organic layers were combined, dried over MgSO4 and evaporated. The obtained oily residue was dissolved in a minimal amount of ethyl acetate and was then purified by silica gel chromatography, using a mixture of hexane and ethyl acetate (1: 3). The filtrate was evaporated and was left to crystallize in the freezer for several hours to give the product (0.33 gram, 83 percent yield) as off-white crystals having a melting point of 67-68 °C.
Example 4

The crude starting material was co-evaporated with toluene again and used without further purification. TES protected dipeptide was dissolved in 38 mL THF (anhydrous, inhibitor- free) and cooled to -45 °C and stirred for 15 minutes before adding KHMDS (0.5 M in toluene, 25.5 mL, 12.8 mmol, 1.1 equiv) drop-wise. After the addition of KHMDS was complete, the reaction mixture was stirred at -45 °C for 15 minutes, and chloromethyl butyrate (1.8 mL, 1.2 equiv, 14 mmol) was added. The reaction mixture changed from light yellow to a bluish color. TLC (20 percent EtOAc/petroleum ether) showed the majority of starting material was converted. LC-MS showed about 7 percent starting material left. The reaction was quenched by adding 3 mL MeOH, the mixture was warmed to room temperature and concentrated under reduced pressure to an oily residue. The residue was dissolved in petroleum ether and passed through short silica plug to remove the potassium salt. The plug was washed with 13 percent EtOAc/petroleum ether, and the collected eluates were combined and concentrated under reduced pressure. The crude alkylated product was passed through an additional silica plug (product/silica = 1:50) and eluted with 13percent EtOAc/petroleum ether to remove residual starting material to give 5.7 g of product. Yield: 76 percent.
References
1. Endocyte I, Vlahov IR, Santhapuram HKR, Kleindl PJ, Leamon CP, You F. Processes for Preparing Tubulysin Derivatives and Conjugates Thereof. WO2013/149185[P], 2013, A1, Page column 102; 104
2. Second Military Medical University. Chen J, Yu N, Zhang Y, Wu C, Gao B, Yao J, Deng L, Ma J. An alprostadil derivatives and its pharmaceutical preparations (by machine translation). CN105777601[P], 2016, A, Paragraph 0078; 0079; 0080; 0081.
3. Ramot At Tel Aviv University Ltd., Bar-Ilan University. Novel Derivatives of Chemotherapeutic Agents and Uses Thereof. WO2005/120577[P], 2005, A2, Page column 52-53
4. Endocyte I, Vlahov IR, Santhapuram HKR, Kleindl PJ, Leamon CP, You F, Processes for Preparing Tubulysin Derivatives and Conjugates Thereof. WO2013/149185[P], 2013, A1, Page column 75-76
- Related articles
- Related Qustion
1,4-Bis-[4-(3-acryloyloxypropyloxy)benzoyloxy]-2-methylbenzene (RM257) is an important organic intermediate to synthetize. The homeotropic alignment liquid crystal film.....
Nov 7,2019Organic Synthesis IntermediateTris is an established basimetric standard and buffer used in biochemistry and molecular biology. It may be used by itself as a buffer or as a component of mixed buffer formulations.....
Nov 7,2019Chemical ReagentsChloromethyl butyrate
33657-49-7You may like
Chloromethyl butyrate manufacturers
- CHLOROMETHYL BUTYRATE
-

- $1.00 / 1kg
- 2019-07-06
- CAS:33657-49-7
- Min. Order: 1kg
- Purity: as customer's need
- Supply Ability: 1000kg