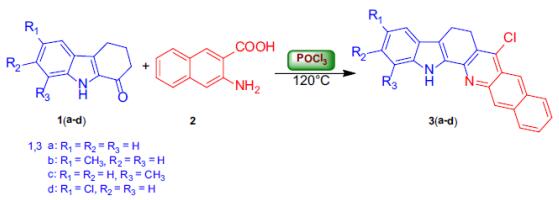
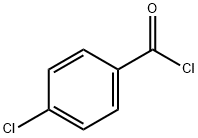
What is 4-Chlorobenzoyl chloride?
Feb 12,2020
4-Chlorobenzoyl Chloride is used as a promoter in the synthesis of α-aminonitriles. It is also used as a derivatization agent and self-assembling dipole molecule to improve hole injection in conjugated polymers. In addition. It is an important organic intermediate to synthetize substituted 4-chlorobenzoyl products.
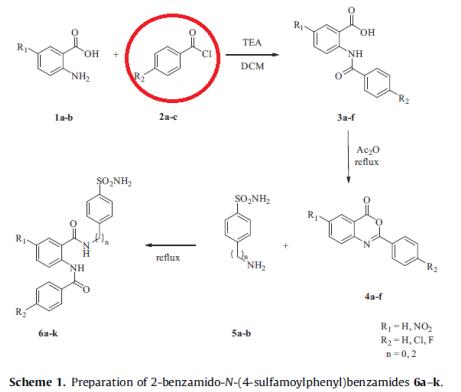
Bozdag et al. reported [1] its application on the synthesis of benzenesulfonamides. They incorporate long, bulky diamide-, 4-oxoquinazoline-3-yl- or quinazoline-4-yl moieties in their molecules, and were investigated for the inhibition of four physiologically relevant CA isoforms, the cytosolic hCA I and II, as well as the transmembrane hCA IX and XII. Most of the new sulfonamides reported here showed excellent inhibitory effects against the four isoforms, with KIs of 7.6–322 nM against hCA I, of 0.06–85.4 nM against hCA II; of 6.7–152 nM against hCA IX and of 0.49–237 nM against hCA XII; respectively.
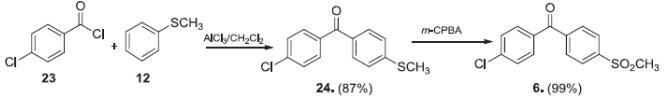
Navarro et al. reported [2] its application on the synthesis of aryl methyl sulfones. It was found that the aryl methyl sulfones based on nonsteroidal anti-inflammatory compounds exhibiting a methyl sulfone instead of the acetic or propionic acid group showed in vitro for inhibition against the human cyclooxygenase of COX-1 and COX-2 isoenzymes and in vivo for anti-inflammatory activity using the carrageenan induced rat paw edema model in rats. Also, in vitro chemosensitivity and in vivo analgesic and intestinal side effects were determined for defining the therapeutic and safety profile.
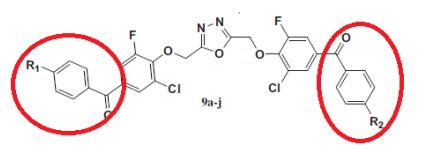
Gurupadaswamy et al. reported [3] its application on the synthesis of 2,5-di(4-aryloylaryloxymethyl)- 1,3,4-oxadiazoles as anti-cancer agents. The cytotoxicity of compounds was evaluated against humanleukemia cell lines (K562 and CEM). The compounds exhibited moderate to good anti-cancer activity. From the investigation, structural activity relationship of these compounds suggests that the position and the type of substituent on the aromatic ring are important for activity. The compounds with chloro group play a dominant role in inhibiting the leukemic cell proliferation.
References
1.Bozdag M. et al. Benzenesulfonamides incorporating bulky aromatic/heterocyclic tails with potent carbonic anhydrase inhibitory activity[J]. Bioorganic & Medicinal Chemistry, 2015, 23:7751–7764
2.Navarro L et al. Synthesis and biological properties of aryl methyl sulfones[J]. Bioorganic & Medicinal Chemistry, 2018, 26:4113–4126
3.Gurupadaswamy HD et al. Synthesis and evaluation of 2,5-di(4-aryloylaryloxymethyl)-1,3,4-oxadiazoles as anti-cancer agents[J]. European Journal of Medicinal Chemistry, 2013, 63:536-543
- Related articles
- Related Qustion
See also
3-Amino-2-naphthoic acid, is an unnatural aromatic amino acid, that can be used for the manufacture of more complex compounds. It can be utilized for the synthesis of novel acronycine/duocarmycin hybrid natural product.....
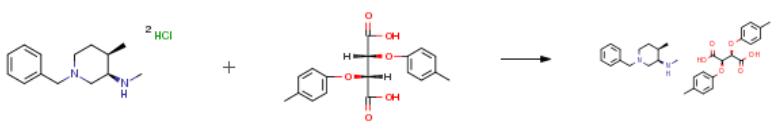
Feb 12,2020Amino Acids and Derivatives(-)-di-p-toluoyl-l-tartaric acid can be used as a chiral resolving agent for the resolution of the racemic bases to isolate the (-)-enantiomeric forms.....
Feb 12,2020Carboxylic acids and derivatives4-Chlorobenzoyl chloride
122-01-0You may like
4-Chlorobenzoyl chloride manufacturers
- 4-Chlorobenzoyl chloride
-

- $10.00 / 1KG
- 2025-05-30
- CAS:122-01-0
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- 4-Chlorobenzoyl chloride
-

- $8.60 / 1KG
- 2025-05-26
- CAS:122-01-0
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: 5000kg
- 4-Chlorobenzoyl chloride
-

- $0.00 / 25Kg/Drum
- 2025-05-16
- CAS:122-01-0
- Min. Order: 1Kg/Bag
- Purity: 99%
- Supply Ability: 20ton