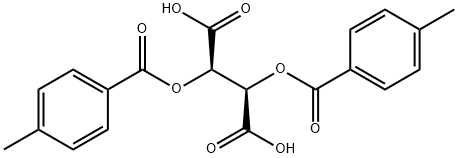
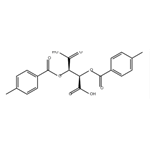
What is (-)-Di-p-toluoyl-L-tartaric acid?
Feb 12,2020
(-)-di-p-toluoyl-l-tartaric acid can be used as a chiral resolving agent for the resolution of the racemic bases to isolate the (-)-enantiomeric forms.
The following example is about its application on the synthesis of (3R,4R)-N,4-dimethyl-1-benzyl-3-piperidinamine sesqui(di-p-toluoyl)-L-tartrate [1].
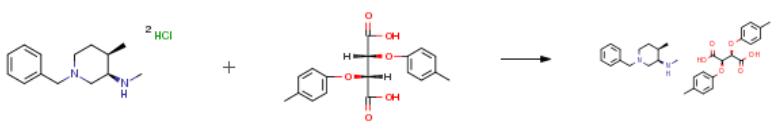
The starting material (1000 g, 3.433 mol) obtained according to the operation of Example 5 was dissolved in a 10percent aqueous sodium hydroxide solution (3000 g). Methyl tert-butyl ether (4000 g) was added, and the mixture was extracted with stirring. The organic phase was separated, and then evaporated and evaporated. The filtrate was collected and concentrated under reduced pressure to give an oil.(720 g, 3.298 mol).The obtained oil was dissolved in ethanol (5800 g), and di-p-toluoyl-L-tartaric acid (640 g, 1.656 mol) was added.Heating is heated to between 60 and 70 ° C, and cooled to between 15 and 25 ° C. A large amount of solids are precipitated and filtered. Collecting solids wherein the enantiomer (3S, 4S)-isomer is about 4percent; The resulting solid was hot beaten with ethanol (5000 g) to give the enantiomer (3S, 4S)-isomer. Yield: 43.0percent.
The following example is about its application on the synthesis of sitagliptin [2].
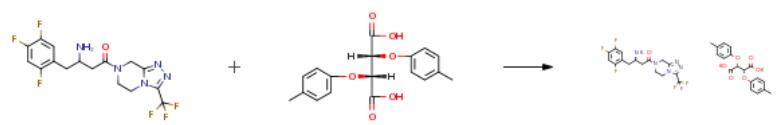
A mixture of methanol (390 ml), water (80 ml), 7-(1-oxo-3-amino-4-(2,4,5- thfluorophenyl)-butyl)-3-thfluoromethyl-5,6,7,8-tetrahydro-1 ,2,4-thazolo[4,3-a]pyrazine (13 g), and di-p-tolyl-L-tartahc acid (13 g) is stirred for about 24 hours. The separated solid is filtered off, washed with ethanol (15 ml) and dried at about 450C to afford the final compound. (Yield: 66.3percent; purity by HPLC: 99.93percent)
The following example is about its application on the synthesis of (+)-5,7-Dichloro-3- {4-[4-(4-chlorophenyl)-piperazin-l- yl]butyl}-3-ethyl-l,3-cHhydro-2/y-indol-2-one [3].
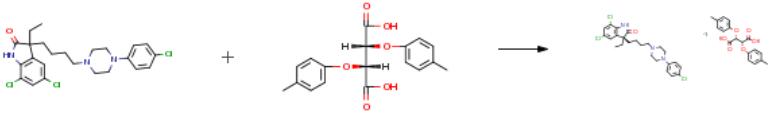
5.00 g (10.40 mmol) racemic 5,7-dichloro-3-{4-[4-(4-chlorophenyl)- piperazin- 1 -yl] -butyl } -3 -ethyl- 1 ,3 -dihydro-2H-indol-2-one base are suspended in 75 ml of ethanol and 4.22 g (10.93 mmol) of di-p-toluyl- tartaric acid are added in portions, while the suspension becomes gradually clear. After complete dissolution, 30 ml of water are added and the mixture is stirred for two hours. After stirring, the precipitated white substance is filtered off, washed with 10 ml portions of the solvent ethanol- water 5:2 (v/v) three times and dried. Yield, 2.60 g (57.6 percent) white powder (diastereomeric salt). The thus obtained diastereomeric salt is added in portions into the stirred solution of 1.4 ml of concentrated ammonia solution in 15 ml of distilled water and subsequently the white suspension is stirred for two hours. The mixture is filtered, washed three times with 3 ml of water each and dried. Yield, 1.42 g (56.8 percent) (+)-5,7-dichloro-3-{4-[4-(4-chlorophenyl)-piperazin- l-yl]-butyl}-3-ethyl-l,3-dihydro-2H-indol-2-one (enantiomeric purity > 99% percent)
References
1.Inner Mongolia Jingdong Pharmaceutical Co., Ltd.; Lv G, Guo R. Tropsch process for cloth intermediate (3 R, 4 R) - N, 4 - dimethyl -1 - benzyl -3 - amino piperdine and its oxalate salt hydrate of new synthetic method (by machine translation). CN109503462[P], 2019, A, Location in patent: Paragraph 0063; 0064
2.Dr. Reddy's Laboratories Limited; Dr. Reddy's Laboratories. Processes for the preparation of sitagliptin and pharmaceutically acceptable salts thereof. INC.WO2009/85990[P], 2009, A2, Location in patent: Page/Page column 29
Egis GNMR, Volk B, Barkóczy J, Gacsalyi I, Fogassy E. Optically active 3-[(phenylpiperazin-1-yl)alkyl]-3-alkyl-oxindole derivatives having CNS activity. WO2010/89616[P], 2010, A1, Location in patent: Page/Page column 20-22
- Related articles
- Related Qustion
See also
4-Chlorobenzoyl Chloride is used as a promoter in the synthesis of α-aminonitriles. It is also used as a derivatization agent and self-assembling dipole molecule to improve hole injection in conjugated polymers....
Feb 12,2020Pharmaceutical intermediatesDi-chlorobis[(1,2,3-)-1-phenyl-2-propenyl]dipalladium(ii) is an important organic regant for the use of transition-metal-mediated organic syntheses.....
Feb 12,2020Catalyst and Auxiliary(-)-Di-p-toluoyl-L-tartaric acid
32634-66-5You may like
- Is benzoic acid polar or nonpolar?
Dec 21, 2023
- Hazards to the Environment and Humans of Ethyl Acetate
Nov 16, 2022
- Ethyl acetate - Uses, Properties, Safety etc.
Dec 29, 2021
(-)-Di-p-toluoyl-L-tartaric acid manufacturers
- (-)-Di-p-toluoyl-L-tartaric acid
-
- $0.00 / 1kg
- 2022-10-15
- CAS:32634-66-5
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 1Ton
- (-)-Di-p-toluoyl-L-tartaric acid
-

- $0.00 / 25Kg/Drum
- 2021-11-04
- CAS:32634-66-5
- Min. Order: 1KG
- Purity: 99%min
- Supply Ability: 10000kg
- (-)-Di-p-toluoyl-L-tartaric acid
-

- $40.00 / 1KG
- 2021-08-12
- CAS:32634-66-5
- Min. Order: 1KG
- Purity: 99
- Supply Ability: 10 tons