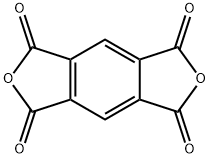
What is Pyromellitic Dianhydride?
Dec 30,2019
Pyromellitic dianhydride (PMDA) is an organic compound with the formula C6H2(C2O3)2. It is the double carboxylic acid anhydride that is used in the preparation of polyimide polymers such as Kapton. It is white crystal powder. The melting point is 286 ℃-288 ℃. When exposed to moisture laden air, it can quickly absorb moisture from the air and hydrolyze into pyromellitic acid [1].
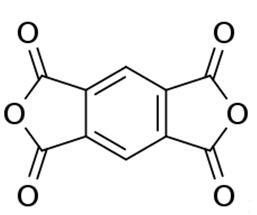
It is prepared by gas-phase oxidation of 1,2,4,5-tetramethylbenzene (or related tetrasubstituted benzene derivatives). An idealized equation is [2]:
C6H2(CH3)4 + 6 O2 → C6H2(C2O3)2 + 6 H2O
In the laboratory, it can be prepared by dehydration of pyromellitic acid using acetic anhydride.
Pyromellitic dianhydride (PMDA) has been used extensively as an important monomer in the
preparation of a variety of thermoplastics such as polyesters, polycarbonates, polyethers polyethers, plasticizersplasticizers, expoxy resins, etc. Moreover, it is also useful in the preparation of high performance coatings that have been widely employed in many fields in our daily life beacuase of its excellent thermal, oxidative stability and excellent mechanical properties. Up to now, many publications in the open literature have been found in synthesis of production of PMDA, and the classical method for such a synthesis constitutes the liquid-phase oxidation of 1,2,4,5-tetraalkyl benzene catalyzed by cobalt, manganese, bromine, nitric acid or dichromic acid in the presence of 100-450 psig, and then dehydrating the obtained pyromellitic acid into pyromellitic dianhydride. However, this procedure is invariably associated with certain limitations such as high cost, long reaction time, environmental hazards, special apparatus and drastic reaction conditions. The catalytic vapor-phase oxidation of 1,2,4,5-tetraalkyl benzene under oxyen pressure using a certain catalyst (e.g. V3O5 TiO2, WO2, V2O5 TiO2) is another well-known method, which, however, requires special equipment, low selectivity, use of stoichiometric and even excess amounts of reagents or catalysts, troublesome work up procedures, etc. Other notable methods to accomplish this conversion include the liquid-phase oxidation of 1,2,4-tetraalkyl benzene, the catalytic vapor-phase oxidation of 1,2,4-tetraalkyl benzene,19-21 etc. However, some of these procedures are invariably associated with one or more disadvantages such as high cost, long reaction time, complicated manufactures special apparatus, etc. Consequently, there is a great need to develop an efficient procedure for the synthesis of pyromellitic dianhydride
Yu Lin Hu etc. reported that pyromellitic dianhydride could be successfully obtained in 76.7% total yield by an aerobic oxidation of 1,4-bis(chloromethyl)-2,5-dimethylbenzene or 1,5-bis(chloromethyl)-2,4-dimethylbenzene catalyzed by Vanadyl acetylacetonate (VO(acac)2)/ copper (II) 2-ethylhexanoate (Cu(2 Eth) 2)/ 1,4-diazabicyclo [2.2.2]octane (DABCO) in [hmim]OTf and a subsequent dehydration of pyromellitic acid upon heating with acetic anhydride. The starting materials including 1,2-bis(chloromethyl)-4,5-dimethylbenzene were prepared by dichloromethylation of their corresponding xylene catalyzed by 1-dodecyl-3-methylimidazolium bromide ([C12mim]Br) in aqueous media [3].
Three-step synthesis of PMDA [3].

PMDA is an electron-acceptor, forming a variety of charge-transfer complexes. It reacts with amines to diimides, C6H2[(CO)2NR]2 which also have acceptor properties [4].
Some evidence suggests that PMDA causes occupational asthma.
Pyromellitic Anhydride (PMDA) is a raw material for heat resistant polyimide resins, films and coatings. PMDA is mainly applied as an intermediate for polyimide films as well as polyimide based composite materials in flexible printed circuit boards, tape automated bonding, magnetic wire insulation, and other high performance applications. It is also used as a curing agent for epoxy resins used in molding powders (to produce components for seal rings, thrust washers, special gaskets or thermal and electrical insulators), adhesives and coatings.
Pyromellitic dianhydride (PMDA) is one of several highly reactive acid anhydrides and used extensively in the production of thermoplastics and high-performance coatings. Anhydrides including PMDA are respiratory irritants and immediate-type sensitisers. Some anhydrides have been associated with occupational asthma. PMDA has also been related to occupational asthma.
Milene Torp Madsen etc. reported three cases with persistent asthmatic symptoms associated with PMDA exposure. Specific inhalation challenge (SIC) suggests PMDA as an asthmogen in all three cases. SIC is considered the reference method for diagnosing asthma caused by low-molecularweight substances.
PMDA had been used at the plant since 2008, to increase the viscosity of plastic food packaging products used in the food industry. Initially, PMDA powder was poured directly in a funnel from 10 kg bags. After a few years, the PMDA bags were opened and added in industrial glove boxes to reduce dust exposure.
In 2014, the company introduced a new larger extruder, where PMDA was added in a semiopen dosing system several metres above the main working area. In the spring of 2015, local exhaust ventilation in the new extruder was closed for repairs. Three employees developed respiratory symptoms within a few months after the new extruder was introduced, particularly when changing filters and during reparations. Respiratory protective equipment was occasionally used during these work processes [5].
References
[1] https://en.wikipedia.org/wiki/Pyromellitic_dianhydride
[2] F. Röhrscheid (2012). "Carboxylic Acids, Aromatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a05_249.
[3] Yu Lin Hu, Ming Lu, Xiao Bin Liu, Sheng Bin Zhang, Zhan Hui Ji, and Ting Ting Lu, An inexpensive and efficient synthetic method for the preparation of pyromellitic dianhydride promoted by ionic liquid, ARKIVOC 2010 (ix) 63-74.
[4] Song, Zhiping; Zhan, Hui; Zhou, Yunhong (2010). "Polyimides: Promising Energy-Storage Materials". Angewandte Chemie International Edition. 49 (45): 8444–8448. doi:10.1002/anie.201002439. PMID 20862664
[5] Milene Torp Madsen, Lars Rauff Skadhauge, Anders Daldorph Nielsen, Jesper Baelum, David Lee Sherson, Pyromellitic dianhydride (PMDA) may cause occupational asthma, Occup Environ Med 2019;76:175–177. doi:10.1136/oemed-2018-105295.
- Related articles
- Related Qustion
- Pyromellitic Dianhydride: Applications in Wastewater Treatment and its Preparation Method May 10, 2024
Pyromellitic Dianhydride, synthesized from 1,2,4,5-Benzenetetracarboxylic Acid, plays a crucial role wastewater treatment.
- Pyromellitic Dianhydride: A Promising Candidate for Creating Functional Carriersbility and Safety Jan 3, 2024
Pyromellitic dianhydride is a white powder with moderate lipophilicity, used to synthesize cyclodextrin polymers for drug delivery, but poses safety hazards.
- Pyromellitic dianhydride: applications and toxicology Jul 20, 2023
Pyromellitic dianhydride has various applications in polymer synthesis, wastewater treatment, solar cell fabrication, but it also has toxicity concerns.
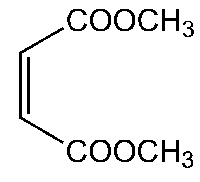
Dimethyl maleate is an organic compound with the formula C6H8O4 and it is the dimethyl ester of maleic acid.....
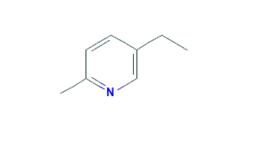
Dec 27,2019Chemical Reagents5-Ethyl-2-methylpyridine is found in alcoholic beverages. 5-Ethyl-2-methylpyridine is present in dry red beans, cocoa, tea and whisky. 5-Ethyl-2-methylpyridine is a flavouring agent.....
Dec 30,2019Flavors and fragrancesPyromellitic Dianhydride
89-32-7You may like
Pyromellitic Dianhydride manufacturers
- Pyromellitic dianhydride
-

- $0.00 / 25Kg/Drum
- 2025-09-30
- CAS:89-32-7
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500mt/year
- 1H,3H-Benzo[1,2-c:4,5-c']difuran-1,3,5,7-tetrone
-
![89-32-7 1H,3H-Benzo[1,2-c:4,5-c']difuran-1,3,5,7-tetrone](/ProductImageEN/2023-04/Small/d45dde99-3bfa-4000-ab5a-3b78cca8b746.jpg)
- $458.00 / 10g
- 2025-09-15
- CAS:89-32-7
- Min. Order: 1g
- Purity: 0.95&0.99
- Supply Ability: 1000
- Pyromellitic Dianhydride
-

- $10.00 / 1KG
- 2025-06-24
- CAS:89-32-7
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt