What is Tributyltin Hydride?
Feb 18,2020
Tributyltin hydride (TBTH) is as a reagent for the reductive ionic 1,5-cyclization of 3-azamuconoates (3-azahexa-2,4-dienedioates). Agafonova reported that a novel 1,5-exo-trig-cyclization of 5-chloro-3-azamuconoates can be used as a base for the development of an effective two-step method for the preparation of 4-hydroxy-1H-pyrrole-3-carboxylic and 3-hydroxy-1H-pyrrole-2,4-dicarboxylic acids derivatives from readily available diazo esters and 2-chloro-2H-azirines or 4-chloroisoxazoles. The experimental results, as well as the DFT calculations of the pyrroles formation, are in good agreement with the ionic mechanism involving the initial hydride attack at the CN bond of the azamuconoate followed by tin-promoted 1,5-cyclization.[1]
Tributyltin hydride can also mediate cyclisation of unsaturated ethers and amines bearing an aldehyde or α,β-unsaturated ketone group[2]. Cyclisation proceeds via reversible addition of the tributyltin radical to the carbonyl double bond to form an intermediate O-stannyl ketyl. This nucleophilic radical can add intramolecularly to electron-rich double bonds to form substituted 5- or 6-membered rings. The efficiency of the cyclisation to form, for example, hydroxytetrahydrofurans, chromanols or quinolones, is depend on the nature of the acceptor double bond and also on the stability of the intermediate O-stannyl ketyl. Thus, resonance-stabilised allylic or benzylic O-stannyl ketyl radicals have been shown, for the first time, to have a particular application in slow radical cyclisations leading to butyrolactones or pyrans. The treatment of tributyltin hydride with α-substituted propenoyl oxazolidinones in the presence of a catalytic amount of AIBN gave the tributyltin radical addition adduct when the substituent is an alkyl group. However, when the substituent is an aryl group, the reduction adduct was obtained in modest diastereoselectivity. The yield of this reduction process was improved by using Pd(PPh3)4 as a catalyst[3].
Tributyltin hydride is also a reducing agent for the reduction of nitroalkenes[3]. The reaction proceeds under almost neutral conditions and works well even in the presence of other reduceable functionalities. Hydrolysis and Nef reaction of the resulting nitronates furnished nitroalkanes and carbonyl compounds respectively in high yields.
It was also found that the nitro groups in tertiary or secondary nitro compounds were replaced by hydrogen or deuterium on treatment with tributyltin hydride or deuteride, respectively. This discovery opened a new area in organic synthesis, as alpha -anions of nitroalkanes can be used as equivalents of alkyl anions. Some new synthetic reactions are devised on the basis of the denitrohydrogenation with Bu//3SnH.
References
1.Agafonova AV, Smetanin IA, Rostovskii NV, Khlebnikov AF, Novikov MS. Expedient synthesis of 3-hydroxypyrroles: Via Bu3SnH-triggered ionic 5- exo-trig -cyclization of 5-chloro-3-azamuconoate derivatives[J]. Organic Chemistry Frontiers, 2018, 5(23):3396 - 3401
2.Bentley Jon, Nilsson PA, Parsons AF. Tributyltin hydride-mediated radical cyclisation of carbonyls to form functionalised oxygen and nitrogen heterocycles[J]. Journal of the Chemical Society. Perkin Transactions 1 (2001), 2002, 12:1461-1469
3.Wu MJ, Fu CL, Duh TH, Yeh JY. Reaction of tributyltin hydride with α,β-unsaturated N-acyloxazolidinones[J]. Synthesis, 1996, 4:462-464.
4.Palomo C, Aizpurua JM, Cossio F, Garcia JM, Lopez MC, Oiarbide M. Tributyltin Hydride Addition to Nitroalkenes: A Convenient Procedure for the Conversion of Nitroalkenes into Nitroalkanes and Carbonyl Compounds[J]. Journal of Organic Chemistry, 1990, 55(7):2070-2078.
5.Ono, MK. Denitrohydrogenation of aliphatic nitro compounds[J]. Yuki Gosei Kagaku Kyokaishi, 1985, 43(2):121-132
- Related articles
- Related Qustion
See also
4-Acetamidobenzenesulfonyl azide is an important organic intermediate (building block) to synthetize substituted amidobenzene products.....
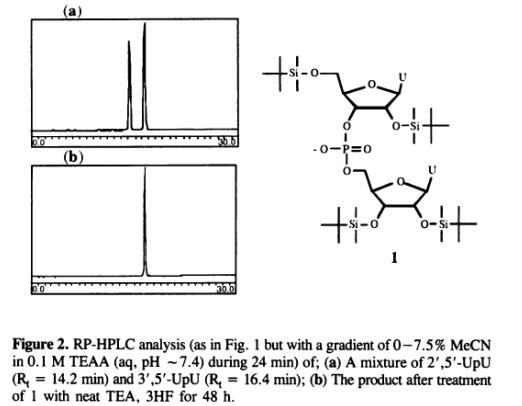
Feb 18,2020Organic Synthesis IntermediateThe t-butyldimethylsilyl (TBDMS) group is the most widely used 2'-OH protection in synthesis of oligoribonucleotides. The TBDMS-group became much used particularly after it was combined with phosphoroamidite methodology.....
Feb 18,2020AmidesTributyltin Hydride
688-73-3You may like
Tributyltin Hydride manufacturers
- Tributyltin Hydride
-

- $1.00 / 1KG
- 2019-07-14
- CAS:688-73-3
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 25kg