Chemicals reagents are commonly referred to as reagent. It is a large class of pure chemical substances with various standard purities and can be used for education, scientific research, analysis and testing as well as the functional materials and raw materials required by some kinds of novel industrial materials. There is a large variety of chemical reagents and the number has reached hundreds of thousands in the world. There are also tens of thousands of reagents that have been used in daily life in china. There is still no uniform international rules in the classification methods for chemicals. It is customary divided by subject and actual application. Take the representative catalog issued by the Germany E • Merck Company as example, there are twelve categories and seventy categories while in most countries, it is divided with the scope of application. We divide the reagents into four categories. 1. General reagents: generally refers to inorganic reagents and organic reagents that can meet standard purity. It is often applied to scientific research, analysis and testing, and synthetic reaction and used as new materials. 2. Analysis Reagents: regent dedicated to analysis and test and can be divided into two subcategories: (1) Reagents for chemical analysis: testing items for the chemical reaction analysis. 1) Baseline Reagent: pure compound directly used for the standard solution in the formulation and volumetric analysis. 2) Indicator: it can be used to indicate the end of the titration reagent and can be classified into pH indicator, redox indicator adsorption indicator, metal indicator, a fluorescent indicator, and so on. (2) Regents for instrument analysis: high-purity compounds dedicated to instrumental analysis. 1) Spectroscopically pure reagents: spectroscopically pure compound, often expressed in the SP for spectral analysis reagents. 2) Chromatography pure reagents: reagents dedicated to analysis of gas chromatography and liquid chrom
Applications of Triethylsilane
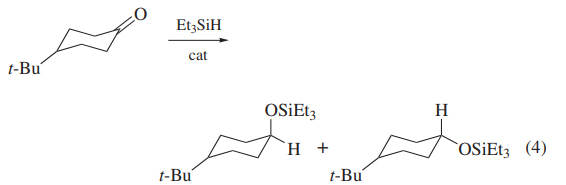
Triethylsilane serves as an exemplar for organosilicon hydride behavior as a mild reducing agent.
Nov 8,2019 Chemical ReagentsToxity of Heptane
Heptane is a chemical derived from petroleum and a member of the alkane series. It is classified as a “straight-chain” alkane. As a chemical, it is a colorless liquid hydrocarbon.
Nov 8,2019 Chemical ReagentsHealth effects of carbon
Carbon is unique in its chemical properties because it forms a number of components superior than the total addition of all the other elements in combination with each other.
Nov 8,2019 Chemical ReagentsDEHP and Endocrine Toxicity
Di-2-ethylhexyl phthalate (DEHP) is the most common member of the class of phthalates, which are used as plasticizers in polymer products to make plastic flexible.
Nov 7,2019 Chemical ReagentsApplications of Tris(hydroxymethyl)aminomethane
Tris is an established basimetric standard and buffer used in biochemistry and molecular biology. It may be used by itself as a buffer or as a component of mixed buffer formulations.
Nov 7,2019 Chemical ReagentsMethod for synthesizing N, O-dimethylhydroxylamine hydrochloride
N,O-Dimethylhydroxylamine is a methylated hydroxylamine used to form so called 'Weinreb amides' for use in the Weinreb ketone synthesis. It is commercially available as its hydrochloride salt.
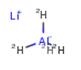
Nov 5,2019 Chemical ReagentsLithium Aluminum Deuteride: Application in Synthetic Works
lithium aluminum deuteride is used as a reducing agent in organic synthesis, especially for the reduction of esters, carboxylic acids, and amides.
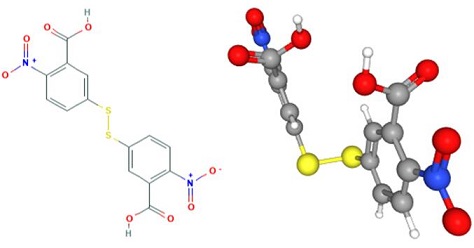
Nov 4,2019 Chemical ReagentsUses of 5,5'-Dithiobis(2-nitrobenzoic acid)
5,5'-Dithiobis(2-nitrobenzoic acid) (DTNB)is an organic disulfide that results from the formal oxidative dimerisation of 2-nitro-5-thiobenzoic acid. An indicator used to quantify the number or concent
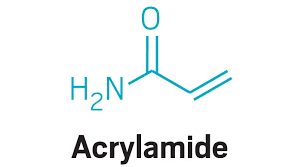
Nov 1,2019 Chemical ReagentsAcrylamide and Cancer Risk
Acrylamide is a substance that forms through a natural chemical reaction between sugars and asparagine, an amino acid, in plant-based foods – including potato and cereal-grain-based foods.
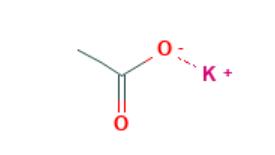
Oct 31,2019 Chemical ReagentsApplications of Potassium Acetate
Potassium Acetate is easily soluble in water, ethanol and methanol, but insoluble in ether. The solution of KOAc is alkaline to litmus but not to p-phenolphthalein. In addition, KOAc is Combustible.
Oct 28,2019 Chemical Reagents