| Identification | Back Directory | [Name]
Cobicistat | [CAS]
1004316-88-4 | [Synonyms]
GS9350
Tybost
GS-9350
GS 9350
Cobiclstat
Cobicistat
Cobicistat D8
Cobicistat (GS-9350)
Thiazol-5-ylmethyl (2R,5R)-5-((S)-2-(3-((2-isopropylthiazol-4-yl)methyl)-3-methylureido)-4-mor
thiazol-5-ylMethyl (2R,5R)-5-((S)-2-(3-((2-isopropylthiazol-4-yl)Methyl)-3-Methylureido)-4-MorpholinobutanaMido)-1,6-diphenylhexan-2-ylcarbaMate
(3R,6R,9S)-12-Methyl-13-[2-(1-methylethyl)-4-thiazolyl]-9-[2-(4-morpholinyl)ethyl]-8,11-dioxo-3,6-bis(phenylmethyl)-2,7,10,12-tetraazatridecanoic acid 5-thiazolylmethyl ester
2,7,10,12-Tetraazatridecanoic acid, 12-Methyl-13-[2-(1-Methylethyl)-4-thiazolyl]-9-[2-(4-Morpholinyl)ethyl]-8,11-dioxo-3,6-bis(phenylMethyl)-, 5-thiazolylMethyl ester, (3R,6R,9S)- | [Molecular Formula]
C40H53N7O5S2 | [MDL Number]
MFCD18251449 | [MOL File]
1004316-88-4.mol | [Molecular Weight]
776.023 |
| Chemical Properties | Back Directory | [Melting point ]
50-54°C | [Boiling point ]
974.5±65.0 °C(Predicted) | [density ]
1.228±0.06 g/cm3 (20 ºC 760 Torr) | [storage temp. ]
Hygroscopic, Refrigerator, under inert atmosphere | [solubility ]
Chloroform (Slightly), DMSO (Slightly), Ethyl Acetate (Slightly), Methanol (Slightly) | [form ]
Solid | [pka]
11.86±0.46(Predicted) | [color ]
White to Off-White | [Stability:]
Hygroscopic | [InChIKey]
ZCIGNRJZKPOIKD-CQXVEOKZSA-N | [SMILES]
C(OCC1SC=NC=1)(=O)N[C@@H](CC1=CC=CC=C1)CC[C@H](CC1=CC=CC=C1)NC(=O)[C@H](CCN1CCOCC1)NC(=O)N(C)CC1=CSC(C(C)C)=N1 |
| Hazard Information | Back Directory | [Uses]
Antiretroviral;Labeled Cobicistat, intended for use as an internal standard for the quantification of Cobicistat by GC- or LC-mass spectrometry. | [Uses]
Cobicistat is a HIV protease inhibitor and have been coadministered with low-dose ritonavir (R535000) as a pharmacoenhancer, significantly increasing their plasma concentrations. | [Uses]
Isotope labelled analogue of Cobicistat (C633150), a HIV protease inhibitor and have been coadministered with low-dose ritonavir (R535000) as a pharmacoenhancer, significantly increasing their plasma
concentrations. | [Originator]
Gilead (United States) | [Definition]
ChEBI: Cobicistat is a monocarboxylic acid amide obtained by formal condensation of the carboxy group of (2S)-2-({[(2-isopropyl-1,3-thiazol-4-yl)methyl](methyl)carbamoyl}amino)-4-(morpholin-4-yl)butanoic acid with the amino group of 1,3-thiazol-5-ylmethyl [(2R,5R)-5-amino-1,6-diphenylhexan-2-yl]carbamate. Acts as a pharmacoenhancer in treatment of HIV-1 by inhibiting P450 enzymes that metabolise other medications.. It has a role as a P450 inhibitor. It is a member of 1,3-thiazoles, a member of morpholines, a member of ureas, a carbamate ester and a monocarboxylic acid amide. | [Brand name]
Tybost | [Clinical Use]
Pharmacokinetic enhancer used to increase the effect of
atazanavir and darunavir | [Synthesis]
Commercial L-methionine (24) was treated with bromoacetic
acid at elevated temperatures to afford aminolactone salt 25 in
70% yield. This material was then reacted with methyl
aminomethylthiazole (26) in the presence of CDI and diisopropylethylamine
to arrive at urea 27 in 91% yield. Next, lactone 27
underwent a ring-opening sequence upon exposure to trimethylsilyl
iodide (TMSI) giving intermediate 28. The iodide was then displaced
by morpholine, followed by treatment with oxalic acid to
deliver the L-thiazole morpholine ethyl ester as the oxalate salt 29 in 71% yield for the sequence. Base-mediated hydrolysis of ethyl
ester 29, followed by treatment of carboxylate 30 with mono-carbonate
hydrochloride 31 in the presence of EDCI and HOBT, provided
cobicistat (V) in 76% yield for two steps.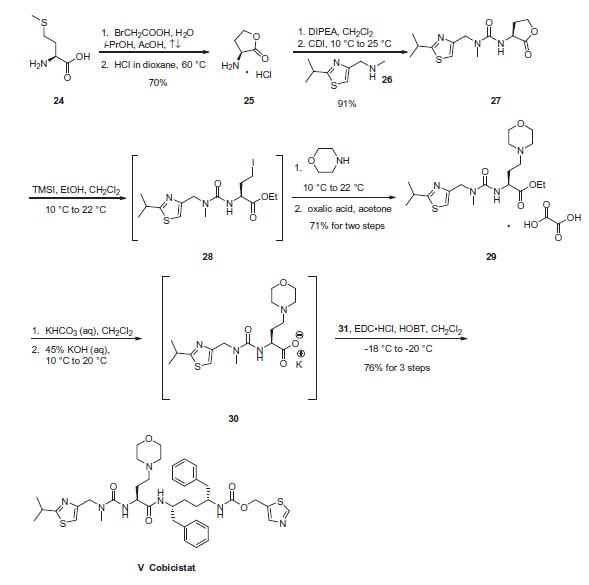
| [Drug interactions]
Potentially hazardous interactions with other drugsAlpha-blockers: concentration of alfuzosin possibly
increased - avoid.
Anti-arrhythmics: concentration of amiodarone
possibly increased - avoid.
Antibacterials: concentration reduced by rifabutin
and rifampicin - adjust cobicistat dose, avoid with
rifampicin.
Anticoagulants: avoid with apixaban; anticoagulant
effect of rivaroxaban possibly enhanced - avoid.
Antidepressants: concentration possibly reduced by
St John’s wort - avoid.
Antiepileptics: concentration of cobicistat
possibly reduced by carbamazepine, fosphenytoin
phenobarbital, phenytoin and primidone - avoid.
Antifungals: concentration of itraconazole and
ketoconazole possibly increased - reduce antifungal
dose.
Antipsychotics: concentration of lurasidone and
pimozide possibly increased - avoid.
Antivirals: concentration of daclatasvir and
maraviroc possibly increased - reduce daclatasvir
and maraviroc dose; avoid with dasabuvir, nevirapine,
ombitasvir, paritaprevir, ritonavir and simeprevir;
concentration of elbasvir and grazoprevir increased -
avoid; concentration of olaparib possibly increased -
avoid or reduce olaparib dose; concentration of both
drugs reduced with tipranavir - avoid.
Anxiolytics: avoid with oral midazolam.
Avanafil: concentration of avanafil possibly increased
- avoid.
Bosentan: avoid concomitant use.
Cardiac glycosides: concentration of digoxin possibly
increased - reduce initial dose of digoxin.
Corticosteroids: concentration of corticosteroids
possibly increased avoid or use with caution.
Cytotoxics: concentration of ibrutinib possibly
increased - reduce ibrutinib dose; concentration of
olaparib possibly increased - avoid or reduce dose of
olaparib.
Domperidone: possible increased risk of ventricular
arrhythmias - avoid.
Ergot alkaloids: concentration of ergot alkaloids
possibly increased - avoid.
Immunosuppression: concentration of ciclosporin,
sirolimus and tacrolimus possibly increased.
Lipid-lowering drugs: concentration of atorvastatin
possibly increased - reduce atorvastatin dose; avoid
with simvastatin.
Oestrogens: metabolism of oestrogens accelerated,
reduced contraceptive effect - avoid or use with
caution.
Salmeterol: avoid concomitant use.
Sildenafil: concentration of sildenafil possibly
increased - avoid sildenafil for pulmonary arterial
hypertension, reduce dose for erectile dysfunction.
Tadalafil: concentration of tadalafil possibly
increased - reduce dose of tadalafil.
Vardenafil: concentration of vardenafil possibly
increased - reduce dose of vardenafil. | [Metabolism]
Cobicistat is metabolised via CYP3A (major)- and
CYP2D6 (minor)-mediated oxidation. Following oral
administration of [14C]-cobicistat, 99% of circulating
radioactivity in plasma was unchanged cobicistat. Low
levels of metabolites are observed in urine and faeces and
do not contribute to the CYP3A inhibitory activity of
cobicistat.
Following oral administration of [14C]-cobicistat, 86%
and 8.2% of the dose were recovered in faeces and urine,
respectively. | [References]
[1] deeks ed. cobicistat: a review of its use as a pharmacokinetic enhancer of atazanavir and darunavir in patients with hiv-1 infection. drugs. 2014 feb;74(2):195-206. |
| Questions And Answer | Back Directory | [Description]
Cobicistat (GS-9350) is a potent and selective inhibitor of CYP3A with IC50 of 30-285 nM. | [In vitro]
Cobicistat (GS-9350) is a potent, and selective inhibitor of human cytochrome P450 3A (CYP3A) enzymes as a pharmacoenhancer. GS-9350 inhibits CYP3A with IC50 spectrum from 30 nM to 285 nM. In contrast to ritonavir, GS-9350 is devoid of anti-HIV activity, with IC50 of > 30μM against HIV-1 protease and EC50 of > 30μM in MT-2 HIV infection assay, and is thus more suitable for use in boosting anti-HIV drugs without risking selection of potential drug-resistant HIV variants. GS-9350 shows reduced liability for drug interactions and may have potential improvements in tolerability over ritonavir. |
|
|