| Identification | Back Directory | [Name]
Duloxetine | [CAS]
116539-59-4 | [Synonyms]
DULOXETIN
DULOXETINE
DULOXETINE-D3
(S)-DULOXETINE
DULOXETINE HCI
Duloxetine Hcl(S)
Duloxetine & Intermediates
methyl-[(3S)-3-(1-naphthyloxy)-3-(2-thienyl)propyl]amine
N-Methyl-gama-(1-naphthalenyloxy)-2-thiophenepropanamine
Methyl[(3S)-3-(naphthalen-1-yloxy)-3-(thiophen-2-yl)propyl]aMine
(3S)-N-methyl-3-naphthalen-1-yloxy-3-thiophen-2-yl-propan-1-amine
(3R)-N-methyl-3-(naphthalen-1-yloxy)-3-thiophen-2-ylpropan-1-amine
(S)-N-Methyl-3-(naphthalen-1-yloxy)-3-(thiophen-2-yl)propan-1-aMine | [EINECS(EC#)]
601-438-0 | [Molecular Formula]
C18H19NOS | [MDL Number]
MFCD06801358 | [MOL File]
116539-59-4.mol | [Molecular Weight]
297.41 |
| Chemical Properties | Back Directory | [Boiling point ]
466.2±40.0 °C(Predicted) | [density ]
1.158±0.06 g/cm3(Predicted) | [storage temp. ]
Store at -20°C | [solubility ]
Soluble in DMSO | [form ]
Oil | [pka]
10.02±0.10(Predicted) | [color ]
Light brown to yellow | [BCS Class]
2 | [InChI]
InChI=1/C18H19NOS.ClH/c1-19-12-11-17(18-10-5-13-21-18)20-16-9-4-7-14-6-2-3-8-15(14)16;/h2-10,13,17,19H,11-12H2,1H3;1H/t17-;/s3 | [InChIKey]
BFFSMCNJSOPUAY-VOPAOICTNA-N | [SMILES]
C1(=CC=CS1)[C@H](CCNC)OC1=CC=CC2=CC=CC=C12.Cl |&1:5,r| | [EPA Substance Registry System]
2-Thiophenepropanamine, N-methyl-?-(1-naphthalenyloxy)-, (?S)- (116539-59-4) |
| Hazard Information | Back Directory | [Chemical Properties]
Duloxetine hydrochloride: Cl8H19NOS·HCl. [136434-34-9]. White solid. The pKa of dimethylformamide-water (66:34) is 9.6. | [History]
Duloxetine is a second-generation antidepressant. Its mechanism of action involves inhibiting the reuptake of serotonin and norepinephrine by neurons, thereby increasing the concentration of these two neurotransmitters in the synaptic cleft, thus improving mood and relieving pain.
Initial Approval (2004): Cymbalta was initially approved for the treatment of major depressive disorder (MDD). In the same year, it also received approval for the treatment of diabetic peripheral neuropathy (DPNP), making it a unique drug capable of simultaneously addressing two common and frequently co-occurring conditions: depression and neuropathic pain.
Expansion of Indications: Subsequently, Eli Lilly expanded its indications through clinical trials, making it a multi-functional drug. The most significant expansions include generalized anxiety disorder (GAD) (2007), fibromyalgia (2008), and chronic musculoskeletal pain (2010). The approval of these indications secured Cymbalta a significant position in the pain management market, making it one of Eli Lilly's blockbuster drugs.
Patent Expiration (2013): In December 2013, Cymbalta's primary patent in the United States expired, a phenomenon known as the "patent cliff."
Following the patent expiration, the FDA quickly approved several generic versions (generic name: duloxetine), leading to a significant drop in sales, but also enabling wider use of the drug at a lower price. | [Uses]
Antidepressant. | [Definition]
ChEBI: (S)-duloxetine is a duloxetine. It is an enantiomer of a (R)-duloxetine. | [Brand name]
Cymbalta (Lilly). | [General Description]
Duloxetine (Cymbalta) is a newer antidepressant. It islargely like venlafaxine, which is an SNERI (selective norepinephrinereuptake inhibitor). | [Pharmacokinetics]
Duloxetine appears to be fairly well absorbed after oral doses, with peak plasma levels in 6 to 10 hours and
linear pharmacokinetics. The drug is extensively metabolized in the liver to active
metabolites, with 72% of an oral dose primarily excreted in the urine as conjugated metabolites and up to
15% appearing in the feces.
N-demethylation to an active metabolite (CYP2D6) and hydroxylation of the naphthyl ring (CYP1A2) at either
the 4-, 5-, or 6-positions are the main metabolic pathways for duloxetine. Its metabolites are primarily
excreted into the urine as glucuronide, sulfate, and O-methylated conjugation products. The
major metabolites found in plasma also were found in the urine. Preclinical data for 4-hydroxyduloxetine
suggests it has a similar pharmacological profile to duloxetine, with selective inhibition of SERT but less
activity at the NET. | [Clinical Use]
Duloxetine has been approved for the treatment of depression and diabetic peripheral
neuropathic pain. It is another analogue in the line of fluoxetine-based products from Lilly, in which the phenyl and phenoxy groups of fluoxetine have been respectively replaced with the benzene isostere,
thiophene, and a
naphthyloxy group (previously described under fluoxetine). Duloxetine exhibits dual inhibition with high
affinity for the SERTs and NETs, with a five times preferential inhibition of the SERT. Duloxetine
appears to be a more potent in vitro blocker of SERTs and NETs than venlafaxine. In humans, duloxetine has
a low affinity for the other neuroreceptors, suggesting low incidence of unwanted adverse effects. | [Synthesis]
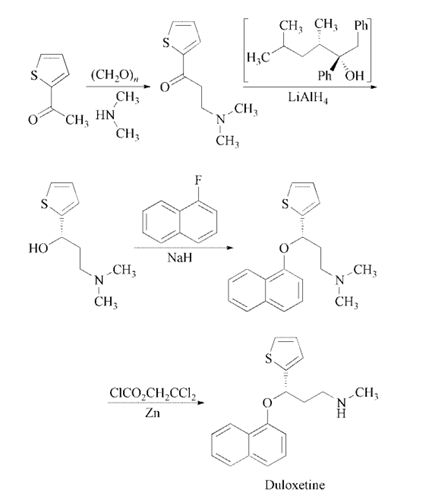
Reaction of 2-acetylthiophene
with paraformaldehyde and dimethylamine in
ethanol gives 3-(dimethylamino)-1-(2-thienyl)-
1-propanone, which is enantioselectively reduced
with a 2:1 complex of (2R,3S)-4-(dimethylamino)-
3-methyl-1,2-diphenyl-2-butanol
and LiAlH4 in toluene to yield (S)-3-(dimethylamino)-
1-(2-thienyl)-1-propanol. The
condensation of (S)-3-(dimethylamino)-1-(2-
thienyl)-1-propanol with 1-fluoronaphthalene
catalyzed by NaH in DMSO affords the corresponding
naphthyl ether (S)-N,N-dimethyl-3-
(naphthalen-1-yloxy)-3-(thiophen-2-yl)propan-
1-amine, which is finally monodemethylated
with 2,2,2-trichloroethyl chloroformate and zinc
in toluene and treated with oxalic acid .
 | [Drug interactions]
Potentially hazardous interactions with other drugs
Antibacterials: metabolism inhibited by ciprofloxacin
- avoid.
Anticoagulants: possibly increased risk of bleeding
with dabigatran.
Other CNS medication: enhanced effect.
Antidepressants: avoid with MAOIs, moclobemide,
St John’s wort, tryptophan, venlaflaxine, amitriptyline,
clomipramine and SSRIs due to increased risk of
serotonin syndrome; increased risk of side effects
with tricyclic antidepressants; fluvoxamine decreases
the clearance of duloxetine by 77% - avoid; possible
increased risk of convulsions with vortioxetine.
Antimalarials: avoid with artemether/lumefantrine
and piperaquine with artenimol.
Dapoxetine: avoid concomitant use.
Methylthioninium: risk of CNS toxicity - avoid if
possible. | [Metabolism]
Duloxetine is extensively metabolised and the metabolites
are excreted principally in urine. Both cytochromes
P450-2D6 and 1A2 catalyse the formation of the two major
metabolites, glucuronide conjugate of 4-hydroxy duloxetine
and sulphate conjugate of 5-hydroxy, 6-methoxy duloxetine.
Based upon in vitro studies, the circulating metabolites of
duloxetine are considered pharmacologically inactive |
|
|