| Identification | Back Directory | [Name]
ABT450 | [CAS]
1216941-48-8 | [Synonyms]
ABT450 5MG
Paritaprevir
ABT450/Paritaprevir
Paritaprevir(ABT-450)
ABT-450;ABT450;ABT 450
Paritaprevir(Veruprevir ABT-450)
Cyclopropa[e]pyrrolo[1,2-a][1,4]diazacyclopentadecine-14a(5H)-carboxamide, N-(cyclopropylsulfonyl)-1,2,3,6,7,8,9,10,11,13a,14,15,16,16a-tetradecahydro-6-[[(5-methyl-2-pyrazinyl)carbonyl]amino]-5,16-dioxo-2-(6-phenanthridinyloxy)-, (2R,6S,12Z,13aS,14aR,16aS)- | [Molecular Formula]
C40H43N7O7S | [MDL Number]
MFCD28411394 | [MOL File]
1216941-48-8.mol | [Molecular Weight]
765.88 |
| Chemical Properties | Back Directory | [density ]
1.45±0.1 g/cm3(Predicted) | [storage temp. ]
4°C, away from moisture and light | [solubility ]
DMSO:30.0(Max Conc. mg/mL);39.2(Max Conc. mM) | [form ]
A solid | [pka]
4.41±0.60(Predicted) | [color ]
White to off-white |
| Hazard Information | Back Directory | [Description]
Paritaprevir hydrate, a second-generation NS3/4A protease
inhibitor, is a component of the all-oral, interferon-free hepatitis
C virus combination therapy developed by Enanta Pharmaceuticals
and AbbVie. The fixed-dose tablet of paritaprevir, ombitasvir
(XXV, NS4A replication complex inhibitor), and ritonavir (cytochrome
P450 inhibitor) taken in combination with dasabuvir (X,
NS5B polymerase inhibitor) was approved for the treatment of
chronic HCV genotype 1 in the USA and EU in 2014, and further approved for treatment of genotype 4 chronic HCV infection
without cirrhosis by the US FDA in 2015. After 12 weeks
of combination treatment, high sustained virological response
rates have been demonstrated in clinical trials.205 Paritaprevir joins
other marketed NS3/4A inhibitors, including telaprevir, boceprevir,
simeprevir, and vaniprevir (XXXVIII), which inhibit a critical enzymatic
complex for HCV replication. It exhibits potent antiviral
activity against HCV genotype 1a and 1b strains, with EC50
values of 1.0 and 0.21 nM respectively. As paritaprevir is metabolized
by CYP3A4, ritonavir, a CYP3A inhibitor with no direct HCV
antiviral properties, is dosed concurrently to boost paritaprevir
exposure, raising the mean plasma half-life to ca. 5.5 h and allowing
for once-daily dosing. While several development routes for
paritaprevir have been published in the patent literature, no process
route has been disclosed to date. Perceptibly the most scalable
route is described below; no yields for this route have been
reported. Notably, the synthesis of a closely related compound
that shares the same macrocylic core has been reported
by AbbVie on kilogram scale. | [Uses]
Paritaprevir is a pharmaceutical drug that is used in the treatment of hepatitis C virus in patients with HCV genotype 1 infection. It inhibits an important viral phosphoprotein, NS5A, which is involved in viral replication, assembly, and secretion. | [Definition]
ChEBI: An azamacrocycle which is used which is in combination with dasabuvir sodium hydrate, ombitasvir and ritonavir (under the trade name Viekira Pak) for treatment of chronic hepatitis C virus genotype 1 infection as well as cirrhosis of the liver. | [Synthesis]
Commercial (2S,4R)-N-Boc-4-hydroxyproline (219)
was reacted with 6-chlorophenanthridine (220) in NMP in the
presence of sodium t-butoxide. Acid 221 was then coupled
with commercial vinylcyclopropylamine fragment 222 using
o-(7-azabenzotriazol-1-yl)-N,N,N0 ,N0-tetramethyluronium hexafluorophosphate
(HATU) and DIPEA to afford peptide 223 following
Boc deprotection. The product could be crystallized upon neutralizing
with NaOH. Amine 223 was subsequently coupled with acid
224 using EDC and N-hydroxy-5-norbornene-2,3-di-carboximide
(HONB) in the presence of N,N-dimethylethylene diamine to afford
linear tripeptide 225. Acid 224 was formed from Boc-
(2S)-amino-non-8-eic acid (229) and 5-methyl-2-pyrazine carboxylic
acid (230) via Boc deprotection and peptide coupling, using
N,N0-disuccinimidyl carbonate and 4-dimethylaminopyridine
(DMAP) to pre-activate acid 230.
Linear trieptide 225 was Boc protected and then subjected to
ring closing metathesis using Zhan-B catalyst (226) in toluene,
using imidazole to quench the catalyst after the reaction. On
kilo-scale, a closely-related ring closing metathesis reaction provided
the desired Z isomer in 61% yield.211 Removal of the Boc carbamate
then provided macrocyclic intermediate 227. Ester
hydrolysis with lithium hydroxide followed by acidification gave
acid 228 which was coupled with cyclopropylsulfonamide (33)
using CDI and 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU). The isolated
product was dissolved in i-PrOAc and diluted with ethanol.
Water was added portion-wise and the solid isolated by filtration
to afford crystalline paritaprevir hydrate (XXVII).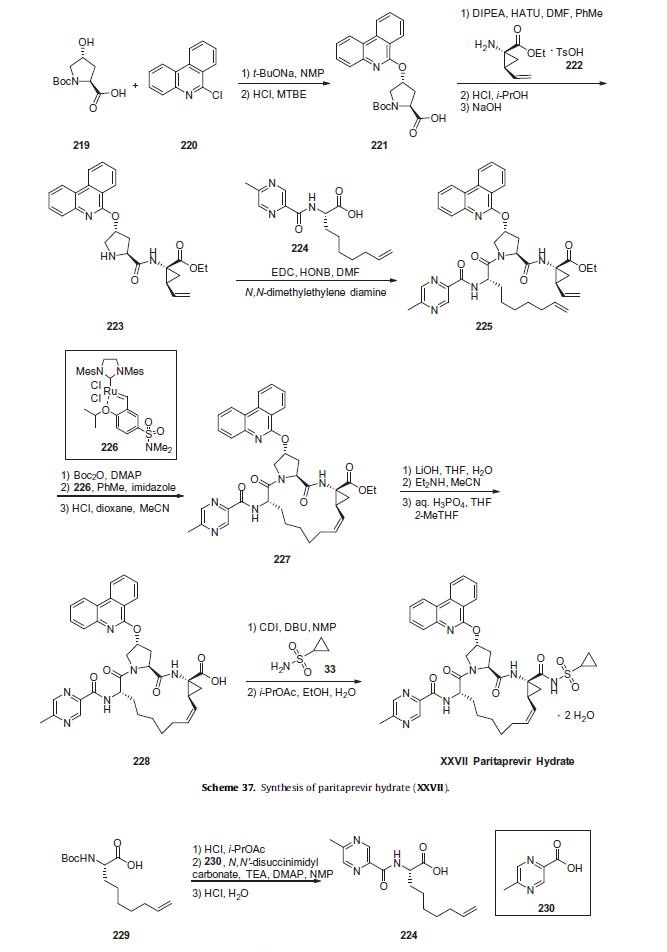
| [storage]
4°C, away from moisture and light |
|
|