| Identification | Back Directory | [Name]
Aripiprazole Lauroxil | [CAS]
1259305-29-7 | [Synonyms]
RDC 3317
1259305-29-7.
Aripiprazole Lauroxil
Dodecanoic acid [7-[4-[4-(2,3-dichlorophenyl)-1-piperazinyl]butoxy]-3,4-dihydro-2-oxo-1(2H)-quinolinyl]methyl ester | [EINECS(EC#)]
826-270-7 | [Molecular Formula]
C36H51Cl2N3O4 | [MDL Number]
MFCD26967976 | [MOL File]
1259305-29-7.mol | [Molecular Weight]
660.71 |
| Chemical Properties | Back Directory | [Melting point ]
83 - 84°C | [Boiling point ]
781.7±60.0 °C(Predicted) | [density ]
1.153±0.06 g/cm3(Predicted) | [storage temp. ]
Refrigerator, under inert atmosphere | [solubility ]
Chloroform (Slightly), Methanol (Slightly, Sonicated) | [form ]
Solid | [pka]
7.67±0.10(Predicted) | [color ]
White to Off-White | [InChIKey]
DDINXHAORAAYAD-UHFFFAOYSA-N | [SMILES]
C(OCN1C2=C(C=CC(OCCCCN3CCN(C4=CC=CC(Cl)=C4Cl)CC3)=C2)CCC1=O)(=O)CCCCCCCCCCC |
| Hazard Information | Back Directory | [Description]
Aripiprazole
lauroxil is a long acting injectable (LAI) pro-drug formulation
of aripiprazole approved in the U.S. for the treatment of
schizophrenia. Aripiprazole lauroxil is a dopamine D2
receptor partial antagonist, a 5-HT2A antagonist, and a 5-
HT1A partial agonist that was developed by Alkermes. It was Aripiprazole
lauroxil is a long acting injectable (LAI) pro-drug formulation
of aripiprazole approved in the U.S. for the treatment of
schizophrenia.28 Aripiprazole lauroxil is a dopamine D2
receptor partial antagonist, a 5-HT2A antagonist, and a 5-
HT1A partial agonist that was developed by Alkermes. It was | [Uses]
Aripiprazole lauroxil, an N-acyloxymethyl proagent of Aripiprazole (HY-14546), is a Long-acting injectable (LAI) typical antipsychotic for schizophrenia and a ligand of dopamine receptor D2R/D4R. Aripiprazole lauroxil is cleaved by body’s enzyme esterase to N-hydroxymethyl aripiprazole (plus lauric acid) and then to aripiprazole (plus formaldehyde), no toxicity. | [Definition]
ChEBI: A dodecanoate ester obtained by formal condensation of the carboxy group of dodecanoic acid with the hydroxy group of 7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}-2-oxo-3,4-dihydroquinolin-1(2H)-yl]methanol. A prodrug for aripipraz
le, it is used for treatment of schizophrenia. | [Synthesis]
The synthesis of aripiprazole lauroxil has only been described
on gram scale in the patent literature. Commercially available aripiprazole (58) was
treated with formaldehyde to give hemiaminal 59 in 65% crude
yield and was then heated with lauric anhydride to give
aripiprazole lauroxil (VII) in 21% overall yield.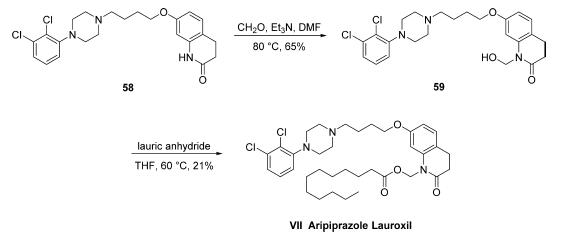
| [in vivo]
Aripiprazole lauroxil (intravenous administration; 1.87 mg/ml) bioconversion in vivo involves the formation of an intermediate, N-hydroxymethyl aripiprazole, the in vitro data indicates a high bioconversion of aripiprazole lauroxil, thus, the concentration of N-hydroxymethyl aripiprazole observed in the animals dosed with aripiprazole lauroxil is surprisingly high[1]. | Animal Model: | Female Sprague Dawley rats[1] | | Dosage: | 1.87?mg/ml? | | Administration: | Blood samples is taken at 5, 15, 30?min and 1, 2, 4, 6, 8 and 24?h after administration | | Result: | Exhibits an clearance: 0.32?±?0.11?L/h/kg.? |
| [References]
[1] Amani P,et al. Characterizing aripiprazole and its ester derivatives, lauroxil and cavoxil, in interaction with dopamine D2 receptor: Molecular docking and dynamics simulations with physicochemical appraisals[J]. Journal of Molecular Liquids, 2022, 362: 119787.
[2] Jann MW, et al. Long-Acting Injectable Second-Generation Antipsychotics: An Update and Comparison Between Agents.CNS Drugs. 2018 Mar;32(3):241-257. DOI:10.1007/s40263-018-0508-6
[3] Rohde M, et al. Biological conversion of aripiprazole lauroxil - An N-acyloxymethyl aripiprazole prodrug.Results Pharma Sci. 2014 May 2;4:19-25. DOI:10.1016/j.rinphs.2014.04.002 |
|
|