| Identification | Back Directory | [Name]
Methscopolamine | [CAS]
13265-10-6 | [Synonyms]
D019832
Methscopolamine
N-Methylhyoscine
METHYLSCOPOLAMINE
n-methylscopolamine
Hyoscine methiodide
Methobromide, hyoscine
(-)-N-Methylscopolamine
Iodide, N-methylscopolamine
Bromide, N-methylscopolamine
1aH,5aH-Tropanium, 6b,7b-epoxy-3a-hydroxy-8-methyl-, (-)-tropate (ester) (8CI)
Tropic acid, (-)-, ester with 6b,7b-epoxy-3a-hydroxy-8-methyl-1aH,5aH-tropanium
(1α,2β,4β,5α)-7β-[(S)-3-Hydroxy-1-oxo-2-phenylpropoxy]-9,9-dimethyl-3-oxa-9-azoniatricyclo[3.3.1.02,4]nonane
3-Oxa-9-azoniatricyclo[3.3.1.02,4]nonane, 7-(3-hydroxy-1-oxo-2-phenylpropoxy)-9,9-dimethyl-, [7(S)-(1a,2b,4b,5a,7b)]-
3-Oxa-9-azoniatricyclo[3.3.1.02,4]nonane, 7-[(2S)-3-hydroxy-1-oxo-2-phenylpropoxy]-9,9-dimethyl-, (1a,2b,4b,5a,7b)- (9CI) | [Molecular Formula]
C18H24NO4+ | [MOL File]
13265-10-6.mol | [Molecular Weight]
318.39 |
| Hazard Information | Back Directory | [Uses]
Methscopolamine inhibits the muscarinic action of acetylcholine on postganlionic para
sympathetic effector regions. It is used for treating stomach ulcers. | [Definition]
ChEBI: Methscopolamine is a 3-hydroxy carboxylic acid. | [Synthesis]
Methscopolamine, 7-(3-hydroxy-1-oxo-2-phenylpropoxy)-9,
9-dimethyl-3-oxa-9-azoniticyclo[3.2.1.0.2,4]nonane nitrate (14.1.7), is synthesized by react�ing scopolamine (14.1.6) with methylbromide and sometimes with a subsequent replacement
of the bromide ion with a nitrate ion by using silver nitrate [9,10].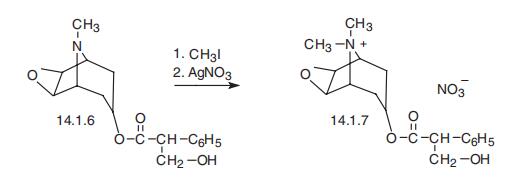
|
|
|