| Identification | Back Directory | [Name]
(S)-(R)-JOSIPHOS | [CAS]
162291-02-3 | [Synonyms]
(S)-(R)-JOSIPHOS
JOSIPHOS SL-J001-2
97% (S)-(R)-JOSIPHOS
(S)1 (1R)2(DIPHENYLPHOSPHINO)FERROCENYL&
(S)-1-[(R)-2-(Diphenylphosphino)ferrocenyl]
(S)1[(1R)2(diphenylphosphino)ferrocenyl] et-dicyclohexylphos
(S)-1-[(1R)-2-(DIPHENYLPHOSPHINO)FERROCENYL]ETHYLDICYCLOHEXYLPHOSPHINE
(S)-1-[(RP)-2-(Diphenylphosphino)ferrocenyl]ethyldicyclohexylphosphine
(S,S)-1-[1-(DICYCLOHEXYLPHOSPHINO)ETHYL]-2-(DIPHENYLPHOSPHINO)FERROCENE
(S)-(+)-1-[(R)-2-(DIPHENYLPHOSPHINO)FERROCENYL]ETHYLDICYCLOHEXYLPHOSPHINE
(S)-(+)-1-[(R)-2-(Diphenylphospino)ferrocenyl] ethylbicyclohexylphosphine
(2S)-1-[(1S)-1-(Dicyclohexylphosphino)ethyl]-2-(diphenylphosphino)ferrocene
(S)-1-[(RP)-2-(Diphenylphosphino)ferrocenyl]ethyldicyclohexylphosphine >=97%
Ferrocene, 1-[(1S)-1-(dicyclohexylphosphino)ethyl]-2-(diphenylphosphino)-, (2S)-
(S)-(+)-1-[(R)-2-(Diphenylphosphino)ferrocenylethyl]dicyclohexylphosphine ethanol adduct
(S)-(+)-1-[(R)-2-(Diphenylphosphino)ferrocenyl]ethyldicyclohexylphosphine ethanol adduct (S)-(R)-JOSIPHOS
(S)-(+)-1-[(R)-2-(Diphenylphosphino)ferrocenyl]ethyldicyclohexylphosphine ethanol adduct,97% (S)-(R)-JOSIPHOS
(S)-(+)-1-[(R)-2-(Diphenylphosphino)ferrocenyl]ethyldicyclohexylphosphineethanoladduct,min.97%(S)-(R)-JOSIPHOS
(S)-(R)-Josiphos, Josiphos SL-J001-2, (2S)-1-[(1S)-1-(Dicyclohexylphosphino)ethyl]-2-(diphenylphosphino)ferrocene (acc to CAS) | [Molecular Formula]
C36H44FeP2 10* | [MDL Number]
MFCD00800285 | [MOL File]
162291-02-3.mol | [Molecular Weight]
594.53 |
| Chemical Properties | Back Directory | [alpha ]
+360° (c 0.5, CHCl3) | [storage temp. ]
Inert atmosphere,2-8°C | [form ]
Powder | [color ]
orange | [Stability:]
store cold | [InChIKey]
HGTBZFMPHBAUCQ-WLOLSGMKSA-N | [SMILES]
[Fe].[CH]1[CH][CH][CH][CH]1.C[C@@H]([C]2[CH][CH][CH][C]2P(c3ccccc3)c4ccccc4)P(C5CCCCC5)C6CCCCC6 |
| Questions And Answer | Back Directory | [Uses]
(S)-(+)-1-[(R)-2-(diphenylphosphine)ferrocene]ethyldicyclohexylphosphine is a Josiphos-class chiral ferrocene phosphine ligand, a very practical and important ligand, second only to BINAP ligands in its pioneering role. It can be used in asymmetric organic synthesis reactions such as asymmetric hydroamination of olefins and Michael addition, and has been widely used in industrial production, playing an important role in the synthesis of many pharmaceuticals and fine chemicals. | [Reactions]
- Ferrocenylphosphine ligands of the type cpFecp(PR2)(*CH(CH3)PR'2) are a class of asymmetric ligands developed at Solvias in Basel, Switzerland. Ligands of this type are currently used industrially in the stereoselective synthesis of commercial products. A unique feature of these bidentate ligands is the presence of a fixed phosphine moiety and a stereogenic,functionalized side chain, which can be easily modified to accommodate electronic and steric requirements. Based on a versatile synthetic procedure starting with optically active ferrocenes of the type cpFecp(PR2)(*CH(CH3)X) [X = OAc or NR2], a variety of donor atoms can be introduced into the side chain.4 These ferrocene based phosphine ligands have wide application in the stereoselective hydrogenation of substituted acetamidoacrylates, enol acetates, β-ketoesters and simple alkenes.
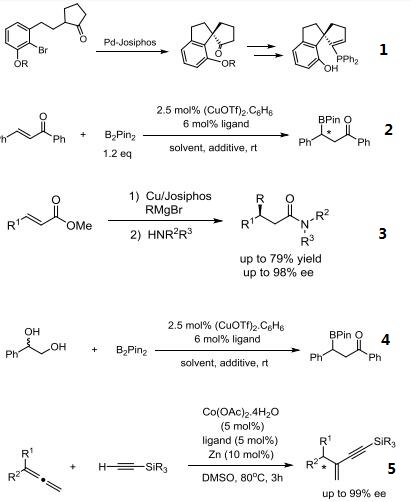
- Pd-catalyzed, enantioselective, intramolecular α-substituted cyclic ketones: facile synthesis of functionalized chiral spirobicycles.
- Asymmetric boron conjugate addition of α,β-unsaturated carbonyl compounds catalyzed by
- CuOTf/Josiphos under non-alkaline conditions.
- Chiral amides via copper-catalyzed enantioselective conjugate addition.
- Ruthenium-catalyzed enantioselective synthesis of β-amino alcohols from 1,2-diols by “borrowing hydrogen”.
- Cobalt-catalyzed asymmetric addition of silylacetylenes to 1,1-disubstituted allenes.
|
|
|