| Identification | Back Directory | [Name]
ISOXADIFEN-ETHYL | [CAS]
163520-33-0 | [Synonyms]
aef122006
Isoxadifen etil
ISOXADIFEN-ETHYL
Isoxadifen ethyl ester
isoxadifen-ethyl(bsi,pa iso)
ETHYL5,5-DIPHENYL-2-ISOXAZOLINE-3-CARBOXYLATE
Isoxadifen-ethyl Solution in Acetonitrile, 1000μg/mL
Ethyl 4,5-Dihydro-5,5-diphenylisoxazole-3-carboxylate
Ethyl 4,5-dihydro-5,5-diphenylisoxazol-3-carboxylate
Ethyl 5,5-diphenyl-4,5-dihydroisoxazole-3-carboxylate
4,5-Dihydro-5,5-diphenyl-3-isoxazolecarboxylic acid ethyl ester
4,5-Dihydro-5,5-diphenylisoxazole-3-carboxylic Acid Ethyl Ester
3-Isoxazolecarboxylic acid, 4,5-dihydro-5,5-diphenyl-, ethyl ester | [EINECS(EC#)]
443-870-0 | [Molecular Formula]
C18H17NO3 | [MDL Number]
MFCD03792846 | [MOL File]
163520-33-0.mol | [Molecular Weight]
295.33 |
| Chemical Properties | Back Directory | [Melting point ]
87-88℃ (ethyl ether ) | [Boiling point ]
407.7±55.0 °C(Predicted) | [density ]
1.15±0.1 g/cm3 (20 ºC 760 Torr) | [vapor pressure ]
0Pa at 20℃ | [storage temp. ]
0-6°C | [form ]
neat | [color ]
White to Almost white | [Water Solubility ]
1.06mg/L at 20℃ | [Major Application]
agriculture
environmental | [InChI]
InChI=1S/C18H17NO3/c1-2-21-17(20)16-13-18(22-19-16,14-9-5-3-6-10-14)15-11-7-4-8-12-15/h3-12H,2,13H2,1H3 | [InChIKey]
MWKVXOJATACCCH-UHFFFAOYSA-N | [SMILES]
O1C(C2=CC=CC=C2)(C2=CC=CC=C2)CC(C(OCC)=O)=N1 | [LogP]
3.8 at 30℃ | [EPA Substance Registry System]
Isoxadifen-ethyl (163520-33-0) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,N | [Risk Statements ]
22-43-50-50/53 | [Safety Statements ]
36/37-61-60-22 | [RIDADR ]
UN3077 9/PG 3 | [WGK Germany ]
3 | [RTECS ]
NY2390000 | [TSCA ]
TSCA listed | [HS Code ]
2934.99.1800 | [HazardClass ]
9 | [PackingGroup ]
III | [Storage Class]
11 - Combustible Solids | [Hazard Classifications]
Acute Tox. 4 Oral
Aquatic Acute 1
Aquatic Chronic 1
Skin Sens. 1 |
| Hazard Information | Back Directory | [Uses]
Isoxadifen-ethyl is a particularly useful pesticide. | [Definition]
ChEBI: An isoxazoline that is the ethyl ester of isoxadifen. It is used as a herbicide safener, especially in conjunction with the herbicides fenoxaprop-P-ethyl and iodosulfuron-methyl-sodium. It is not approved for use within the European Union. | [Preparation]
Isoxadifen-ethyl is prepared by reacting 1, 1-diphenyl ethylene and ethyl 2-chloro-2-(hydroxyimino)acetate under the action of alkali metal compounds. The reaction process is as follows:
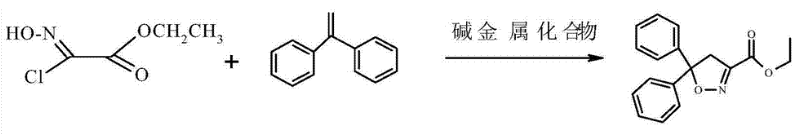 | [Flammability and Explosibility]
Nonflammable | [Synthesis]
Ethyl 2,2-diphenylcyclopropanecarboxylate (0.97 g, 3.7 mmol) was dissolved in 3.7 mL of trifluoroacetic acid (CF3CO2H). Sodium nitrite (NaNO2, 0.28 g, 4.0 mmol, 1.1 eq.) was added to the solution in batches under controlled reaction temperature not exceeding 40 °C. After addition, the reaction mixture was stirred at room temperature (about 18 °C) for 30 minutes. Subsequently, the reaction mixture was poured into ice water and extracted with ether (2 x 20 mL). The organic phases were combined and washed sequentially with saturated aqueous sodium bicarbonate (NaHCO3) (2 x 20 mL), water (20 mL) and brine (20 mL). The washed organic phase was dried with anhydrous sodium sulfate (Na2SO4). After filtration to remove the desiccant, the organic phase was concentrated under reduced pressure to give a light brown oily crude product. 1H NMR analysis of the crude product showed complete reaction with 100% yield. The crude product was further purified by silica gel column chromatography (Rf: 0.45, eluent: petroleum ether solution of 20% ethyl acetate) to afford ethyl 5,5-diphenyl-4,5-dihydroisoxazole-3-carboxylate (0.958 g, 89% yield) as a white solid.1H NMR (400 MHz, CDCl3): δ 7.41-7.26 (m, 10H), δ 4.34 (q, J = 7.1 Hz, 2H), 3.86 (s, 2H), 1.36 (t, J = 7.1 Hz, 3H). 13C NMR (400 MHz, CDCl3): δ 160.54, 151.09, 142.99, 128.54, 128.02, 125.95, 94.79, 62.15, 46.78 14.12. | [References]
[1] Patent: US2016/60223, 2016, A1. Location in patent: Paragraph 0022 |
|
|