| Identification | Back Directory | [Name]
Naltrexone | [CAS]
16590-41-3 | [Synonyms]
Um-792
C07253
en1639
en1939
trexan
Naltrel
NeMexin
celupan
Trexonil
Vivitrex
Vivitrol
Depotrex
NALTREXONE
NALTREXONE10G
NaltrexoneBase
Naltrexone impurty
Naltrexone solution
Naltrexone (200 mg)
Naltrexone USP/EP/BP
Naltrexone, 1.0 mg/mL
Naltrexone Base & HCL
Naltrexone (controlled) HCl
Naltrexone Base Monohydrate
Naltrexone (base, anhydrous)
Naltrexone trifluoroacetate salt
n-cyclopropylmethylnoroxymorphone
Naltrexone (1.0 mg/mL in Methanol)
Naltrexone Hydrochloride Impurity 1
Naltrexone (base and/or unspecified salts)
n-cyclopropylmethyl-14-hydroxydihydromorphinone
17-(cyclopropylmethyl)-4,5-epoxy-3,14-dihydroxymorphinan-6-one
3,14-Dihydroxy-17-(cyclopropylmethyl)-4,5α-epoxymorphinan-6-one
4,5α-Epoxy-3,14β-dihydroxy-17-(cyclopropylmethyl)morphinan-6-one
(5α)-17-(Cyclopropylmethyl)-4,5-epoxy-3,14-dihydroxymorphinan-6-one
17-(cyclopropylmethyl)-4,5-alpha-epoxy-3,14-dihydroxy-morphinan-6-on
5-epoxy-3,14-dihydroxy-17-(cyclopropylmethyl)-(5-alpha)-morphinan-6-on
Morphinan-6-one,17-(cyclopropylMethyl)-4,5-epoxy-3,14-dihydroxy-, (5a)-
Morphinan-6-one, 17-(cyclopropylmethyl)-4,5-epoxy-3,14-dihydroxy-, (5α)-
(4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-dihydroxy-2,3,4,4a,5,6-hexahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinolin-7(7aH)-one | [EINECS(EC#)]
240-649-9 | [Molecular Formula]
C20H23NO4 | [MDL Number]
MFCD00242996 | [MOL File]
16590-41-3.mol | [Molecular Weight]
341.4 |
| Chemical Properties | Back Directory | [Melting point ]
168-170° | [Boiling point ]
477.03°C (rough estimate) | [density ]
1.2064 (rough estimate) | [refractive index ]
1.5614 (estimate) | [Fp ]
9℃ | [storage temp. ]
2-8°C | [solubility ]
Chloroform (Slightly), Methanol (Slightly) | [form ]
Solid | [pka]
pKa 8.38/8.13(H2O,t =20/37,I<0.01) (Uncertain) | [color ]
White to Light Beige | [Major Application]
forensics and toxicology | [InChI]
InChI=1/C20H23NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,15,18,22,24H,1-2,5-10H2/t15-,18+,19+,20-/s3 | [InChIKey]
DQCKKXVULJGBQN-AOJBWHFRNA-N | [SMILES]
[C@@]123CCN(CC4CC4)[C@@H]4CC5=CC=C(O)C(O[C@H]1C(CC[C@]24O)=O)=C35 |&1:0,8,17,21,r| | [EPA Substance Registry System]
Morphinan-6-one, 17-(cyclopropylmethyl)-4,5- epoxy-3,14-dihydroxy-, (5.alpha.)-(16590-41-3) |
| Hazard Information | Back Directory | [Description]
This drug does not have agonistic properties. It is similar to naloxone in terms of pharma�cological characteristics; however, it differs in two important ways—long-lasting action
and that its metabolite 6-β-naltrexol is also a strong antagonist. Naltrexone is potentially
hepatotoxic. Naltrexone is used for blocking pharmacological effects of opioids upon their
overdose. | [Originator]
Antaxone,Zambon Group,Italy | [History]
Naloxone was discovered by Drs. Jack Fischman and Mozez Lewenstein of the Memorial Sloan Kettering Institute for Cancer Research in 1961, based on a theory proposed by their colleague, Dr. Harold Blumberg at the Long Island-based Endo Laboratories. Intravenous naloxone (Envizio) was approved by the Food and Drug Administration (FDA) for opioid overdose reversal in 1971 and was shortly adopted as a standard emergency treatment at many of the nation’s premier academic medical centers.
| [History]
Naltrexone was first synthesized in 1963 by Endo Laboratories, which was patented by Endo Laboratories in 1967 under the developmental code name EN-1639A. Naltrexone was later purchased in 1969 by DuPont Pharmaceuticals. It was developed by the National Institute on Drug Abuse in the 1970s and early 1980s . In 1984, it was approved by the Food and Drug Administration (FDA) for the treatment of heroin addiction . In 1995, Naltrexone was approved by the FDA for the treatment of alcoholism, when the brand name was changed by DuPont to Revia. A new extended-release formulation of naltrexone has been developed and was approved by the FDA in 2006 for use in the treatment of alcohol dependence. | [Uses]
anthelmintic, teniacide | [Uses]
Labeled Naltrexone, intended for use as an internal standard for the quantification of Naltrexone by GC- or LC-mass spectrometry. | [Definition]
ChEBI: An organic heteropentacyclic compound that is naloxone substituted in which the allyl group attached to the nitrogen is replaced by a cyclopropylmethyl group. A mu-opioid receptor antagonist, it is used to treat alcohol dependence. | [Indications]
Naltrexone, an orally active opioid receptor antagonist,
restores erectile function in some patients with idiopathic
ED. | [Manufacturing Process]
Codeine is a component of gum opium and can also be produced by
methylation of morphine using known prior art techniques.
A solution of codeine (30 g, 100.2 mmol), acetic anhydride (18.4 g, 180.2
mmol), triethylamine (18.25 g, 180.2 mmol) and 4-dimethylaminopyridine
(0.5 g) in dry ethyl acetate (620 ml) was stirred at rt. under nitrogen for 12
hr, added saturated aqueous sodium bicarbonate solution until no acetic
anhydride detected. The organic portion was separated, washed with water (3times 120 ml), dried over anhydrous sodium sulfate, and evaporated in vacuo
to dryness to give 6-acetylcodeine as white solids (34.0 g, 99% yield).
Preparation of 6-acetylnorcodeine hydrochloride.
A solution of 6-acetylcodeine (10.0 g, 29.3 mmol), 1-chloroethyl
chloroformate (5.51 g, 37.8 mmol), and proton sponge (1.0 g) in methylene
chloride (80 ml) was heated at reflux for 80 min. The reaction mixture was
evaporated in vacuo to dryness. The residue was chromatographed on silica
gel with ethyl acetate to give 6-acetyl-17-(1-chloroethoxycarbonyl)norcodeine
as an oil (12.13 g), which was dissolved in methanol with a few drops of conc.
HCl. The solution was heated at reflux for 1 hr and evaporated in vacuo to
almost dryness. The residue was added hexane and filtered to give 6-
acetylnorcodeine hydrochloride (10.7 g, 100% yield).
Preparation of norcodeine hydrochloride.
A solution of 6-acetylcodeine (10.0 g, 29.3 mmol), 1-chloroethyl
chloroformate (5.56 g, 38.1 mmol), and proton sponge (1.0 g) in methylene
chloride (50 ml) was heated at reflux for 50 min. The reaction mixture was
evaporated in vacuo to about 30 ml. Methanol (25 ml) and concentrated HCl
(2 ml) were added. The solution was heated at reflux for 40 min. and
evaporated in vacuo to almost dryness. The residue was added hexane and
filtered to give norcodeine hydrochloride (8.8 g, 93% yield).
Preparation of 17-cyclopropylmethylnorcodeine.
A mixture of norcodeine hydrochloride (11.48 g, 27.8 mmol),
(chloromethyl)cyclopropane (5.14 g, 55.6 mmol), sodium carbonate (14.73 g,
139.0 mmol), and potassium iodide (4.61 g, 27.8 mmol) in ethanol (250 ml)
was heated at reflux for 20 hr, cooled, and evaporated in vacuo to dryness.
The residue was basified with NH4OH, and extracted with methylene chloride.
The extract was washed with water and evaporated in vacuo to dryness. The
residue (11.7 g) was chromatographed on silica gel with a eluting solvent
system of methanol/ethyl acetate (10/90) to give 17-
cyclopropylmethylnorcodeine (10.68 g, 91% yield).
Preparation of 17-cyclopropylmethylnorcodeinone.
To a solution of DMSO (14.50 g, 185.6 mmol) in methylene chloride (80 ml)
at -78°C, was added a solution of oxalyl chloride (11.78 g, 92.8 mmol) in
methylene chloride (20 ml) in 20 min. After stirring at -78°C for 20 min., a
solution of 17-cyclopropylmethylnorcodeine (9.0 g, 26.5 mmol) in methylene
chloride (40 ml) was added dropwise in 50 min. The reaction mixture was
stirred at -74° to -76°C for 3 hr, added triethylamine (9.39 g, 92.8 mmol),
allowed to warm up to rt., added methylene chloride (200 ml), washed with
water (10 times 50 ml), and evaporated in vacuo to dryness. The residue was
mixed with hexane and filtered to give 17-cyclopropylmethylnorcodeinone
(8.85 g, 99% yield).
Preparation of 17-cyclopropylmethylnorcodeinone dienol acetate.
A mixture of 17-cyclopropylmethylnorcodeinone (3.55 g, 10.5 mmol), acetic anhydride (20 ml, 210.4 mmol), sodium acetate (1.3 g, 15.8 mmol), and
toluene (6 ml) was heated at 71°-73°C for 14 hr. The reaction mixture was
cooled, added methylene chloride (250 ml), water (50 ml), and sodium
bicarbonate (73.5 g), stirred for 4 hr, and filtered. The organic portion of the
filtrate was separated, washed with water (30 ml), dried over anhydrous
sodium sulfate, and evaporated in vacuo to dryness. The residue (3.94 g) was
chromatographed on silica gel with 100% ethyl acetate to give 17-
cyclopropylmethylnorcodeinone dienol acetate (2.87 g, 72% yield).
Preparation of 17-cyclopropylmethyl-14-hydroxynorcodeinone. A solution of
17-cyclopropylmethylnorcodeinone (0.20 g, 0.59 mmol), formic acid (90%,
0.304 g), water (0.504 g), EtOAc (0.27 g), and hydrogen peroxide (30%, 0.17
g) was heated at 42°-43°C for 15 hr, added water (20 ml), basified with
Na2CO3 (1.02g), and extracted with EtOAc (80 ml and 2 times 20 ml). The
combined extract was washed with water, dried over anhydrous sodium
sulfate, and evaporated in vacuo to dryness to give 17-cyclopropylmethyl-14-
hydroxynorcodeinone (0.10 g, 56% yield). The Rf value in TLC and the IR
spectrum of the product were comparable to those obtained from an authentic
sample.
Preparation of 17-cyclopropylmethyl-14-hydroxynorcodeinone.
A solution of 17-cyclopropylmethylnorcodeinone dienol acetate (1.00 g, 2.63
mmol), formic acid (8 ml, 90%), and hydrogen peroxide (0.37 g, 30%, 3.26
mmol) was heated at 44°-45°C for 6 hr, added water (20 ml) and ethyl
acetate (80 ml), basified with sodium bicarbonate. The organic portion was
separated, washed with water (15 ml), dried over anhydrous sodium sulfate
and evaporated in vacuo to dryness, the residue (0.9 g) was chromatographed
on silica gel with methanol/methylene chloride (2.5/97.5) to give 17-
cyclopropylmethyl-14-hydroxynorcodeinone (0.72 g, 78% yield).
Preparation of 17-cyclopropylmethyl-14-hydroxynorcodeinone.
A solution of 17-cyclopropylmethylnorcodeinone dienol acetate (0.5 g, 1.31
mmol), 3-chloroperbenzoic acid (0.36 g, 2.10 mmol) and oxalic acid (0.27 g,
2.90 mmol) in acetic acid (7 ml) was stirred at rt. overnight, added cold water
(35 ml), basified with sodium carbonate, and extracted with methylene
chloride (100 ml). The extract was washed with water (2 times 30 ml), dried
over anhydrous sodium sulfate, and evaporated in vacuo to dryness. The
residue (0.41 g) was chromatographed on silica gel to give 17-
cyclopropylmethyl-14-hydroxynorcodeinone (0.34 g, 74% yield). The Rf value
in TLC and the IR spectrum of the product were comparable to those obtained
from an authentic sample.
Preparation of 3-methylnaltrexone.
A mixture of 17-cyclopropylmethyl-14-hydroxynorcodeinone (0.30 g, 0.85
mmol) and Pd/C (5%, 0.45 g) in ethanol (35 ml) was hydrogenated in a Parr
hydrogenator at rt. under 28 psi of hydrogen gas. The mixture was filtered.
The filtrate was evaporated in vacuo to dryness to give 3-methylnaltrexone
(0.30 g, 99% yield).
Preparation of naltrexone from 3-methylnaltrexone.
A solution of 3-methylnaltrexone (0.48 g, 1.35 mmol) in methylene chloride
(30 ml) was cooled with an ice-water bath, and then added a solution of
boron tribromide (5.4 ml, 1 M solution in methylene chloride, 5.4 mmol). The
reaction mixture was stirred at rt. for 15 hr, basified with NH4OH, and
extracted with methylene chloride (60 ml). The extract was washed with water
(2 times 15 ml), dried over anhydrous sodium sulfate, and evaporated in
vacuo to dryness to give naltrexone (0.45 g, 98% yield). | [Brand name]
Vivitrol (Alkermes). | [Therapeutic Function]
Narcotic analgesic | [Biological Functions]
Naltrexone (Trexan) is three to five times as potent as
naloxone and has a duration of action of 24 to 72 hours,
depending on the dose. It is used orally in the treatment
of opioid abstinence. Naltrexone exhibits a large firstpass
effect in the liver. However, the major metabolite,
6-β-naltrexol, is also a pure opioid antagonist and contributes
to the potency and duration of action of naltrexone.
Administration of naltrexone orally blocks the
subjective effects of abused opioids and is used to decrease
the craving for opioids in highly motivated recovering
addicts. However, high doses of the opioids
can overcome the naltrexone blockade and lead to
seizures or respiratory depression and death. In addition,
it has been reported recently that naltrexone can
reduce the craving for alcohol in alcoholic patients.
Naltrexone also has been used with success in treating
apneic episodes in children, an effect hypothesized to
be due to blockade of β-endorphin–induced respiratory
depression.
Naltrexone can induce hepatotoxicity at doses only
five times the therapeutic dose and should be used with
care in patients with poor hepatic function or liver damage.
Side effects of the use of naltrexone are more frequently
observed than following naloxone administration.
Such side effects include headache, difficulty
sleeping, lethargy, increased blood pressure, nausea,
sneezing, delayed ejaculation, blurred vision, and increased
appetite. | [General Description]
Naltrexone is a pure opioid antagonist at allopioid receptor subtypes with the highest affinity for theμ-receptor. Naltrexone is orally bioavailable and blocksthe effects of opiate agonists for approximately 24 hoursafter a single dose of 50 mg. It produces no opioid agonisteffects and is devoid of any intrinsic actions other thanopioid receptor blockade. Theoretically, it should workwell to treat opioid dependence but in clinical practice,patients have shown poor compliance and high relapserates. Naltrexone has also been studied to treat alcohol dependencewith mixed results. To address the complianceissues and effectively remove the “choice” of taking theantagonist, naltrexone was developed into an extendedreleaseinjectable microsphere formulation for IM injectiononce a month (Vivitrol). This formulation providessteady-state plasma concentrations of naltrexone threefoldto fourfold higher than the 50-mg oral dose 4 times aday. Currently, Vivitrol is only indicated for the treatmentof alcohol dependence. A Cochrane review found insufficientevidence from randomized controlled trials toevaluate its effectiveness for treating opioid dependence. Currently, phase II and phase III clinical trials ofan implantable pellet form of naltrexone are being conductedfor treating opioid dependence.
The CYP450 system is not involved in naltrexonemetabolism. Naltrexone is reduced to the active antagonist6-β-naltrexol by dihydrodiol dehydrogenase, a cytosolicenzyme. Naltrexone has a black box warning, because ithas the potential to cause hepatocellular injury when givenin excessive doses. | [Biological Activity]
Naltrexone is derived from oxymorphone and exhibit agonist activity only at doses that are of little clinical significance. In the absence of opioid drugs, naloxone does not cause analgesia, respiratory depression, or sedation. However, when administered with an opioid analgesic, the effects produced by the opioid agonist are promptly reversed. The ability to antagonize opioids at all of the different opioid receptors makes naloxone useful for the treatment of opioid overdose. Naltrexone has a similar profile, but it is orally active and has a significantly longer half-life. | [Clinical Use]
Naltrexone is a pure opioid antagonist and has no
analgesic activity. Naltrexone has a higher intravenous
potency and longer duration of action
than naloxone. It has a higher oral bioavailability
and is given by mouth to treat opioid dependence
and to maintain abstinence during opioid detoxification
. In opioid-dependent persons, naltrexone
induces an acute withdrawal reaction. | [Synthesis]
Naltrexone, (-)-17-(cyclopropylmethyl)-4,5-epoxy-3,14-dihydroxymorphi�nan-6-one (3.1.93), is an N-cyclopropylmethyl derivative of oxymorphone (3.1.82). One of
the methods of synthesis is analogous to the synthesis of naloxone, which consists of using
cyclopropylmethylbromide instead of allylbromide.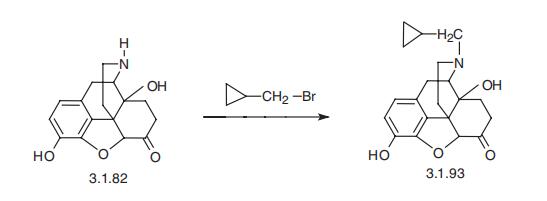
|
|
|