| Identification | Back Directory | [Name]
O-(2,4-dinitrophenyl)hydroxylamine | [CAS]
17508-17-7 | [Synonyms]
NSC 148499
2,4-NitrophenoxyaMine
2,4-DinitrophenoxyaMine
4-dinitrophenyl)hydroxylamine
2,4-Dinitrophenylhydroxylamine
1-aMinooxy-2,4-dinitro-benzene
O-(2,4-dinitrophenyl)hydroxylamine
HydroxylaMine,O-(2,4-dinitrophenyl)- | [EINECS(EC#)]
241-512-6 | [Molecular Formula]
C6H5N3O5 | [MDL Number]
MFCD00075001 | [MOL File]
17508-17-7.mol | [Molecular Weight]
199.121 |
| Chemical Properties | Back Directory | [Melting point ]
112.5°C | [Boiling point ]
336.62°C (rough estimate) | [density ]
1.6937 (rough estimate) | [refractive index ]
1.6910 (estimate) | [storage temp. ]
Keep in dark place,Sealed in dry,2-8°C | [solubility ]
DMSO (Sparingly), Methanol (Slightly) | [form ]
solid | [pka]
-1.07±0.70(Predicted) | [color ]
yellow | [InChI]
InChI=1S/C6H5N3O5/c7-14-6-2-1-4(8(10)11)3-5(6)9(12)13/h1-3H,7H2 | [InChIKey]
YLACRFYIUQZNIV-UHFFFAOYSA-N | [SMILES]
NOC1=CC=C([N+]([O-])=O)C=C1[N+]([O-])=O |
| Questions And Answer(Q&A) | Back Directory | [Preparation]
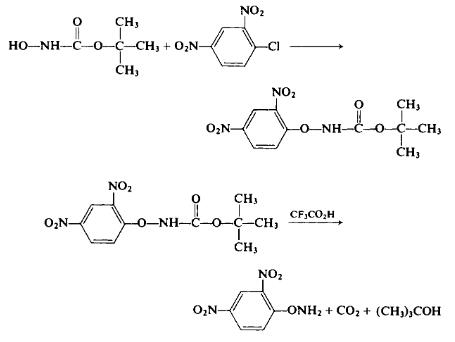
To a stirred solution of 13.3 gm (0.1 mole) of t-butyl JV-hydroxycar-bamate and 5.6 gm (0.1 mole) of potassium hydroxide in 200 ml of absolute ethanol is added 20.2 gm (0.1 mole) of 2,4-dinitrochlorobenzene. The resultant deep red solution is stirred at room temperature for 1 hr; then enough glacial acetic acid is added dropwise to produce a light yellow solution. The solution is poured into 1.5 liters of cold water. The yellow oil which separates is gradually converted to crystals. The solid ί-butyl JV-(2,4-dinitrophenoxy)carbamate is separated, dried, and recrystallized from an ethyl acetate-hexane mixture to afford 16.4 gm (53%), m.p. 74-75°C. To 15 ml of trifluoroacetic acid is added 4 gm (0.0133 mole) of the i-butyl J/V-(2,4-dinitrophenoxy)carbamate. After the evolution of carbon dioxide has subsided, the solution is poured into 100 ml of ice water. The resultant oily layer crystallizes on standing to afford 2.5 gm (95%), m.p. 112°C (from ethanol).
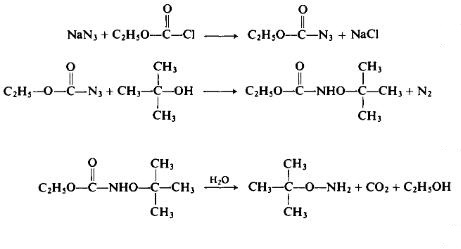
Recently it was discovered that the alkylation of ethyl JV-hydroxy-carbamate under alkaline conditions, particularly in a DMF medium at 60°C in the presence of sodium bicarbonate, leads to the ultimate formation of O-alkylated hydroxylamines. On the other hand, at 80-85°C, the direct alkylation without the presence of a base ultimately leads to N-alkylhydrox-ylamines (see Table I) [59]. The reaction of ethylazidoformate with an alcohol, while perhaps hazardous, may have some merit (Eqs. 31-33). The overall yield, based on ethyl chloroformate, is said to be on the order of 60%.
 |
| Hazard Information | Back Directory | [Chemical Properties]
Light yellow solid | [Uses]
Efficient agent for metal-free amination of arylboronic acids leading to primary anilines. Reagents used in Rhodium-catalyzed aziridines formation. | [Uses]
O-(2,4-Dinitrophenyl)hydroxylamine is a rapid active-site-directed inhibitor of D-amino acid oxidase; modification results in specific incorporation of an amine group into an accessible nucleophilic r
esidue with concomitant release of 2,4-dinitrophenol. | [Preparation]
To a stirred solution of 13.3 gm (0.1 mole) of t-butyl JV-hydroxycar-bamate and 5.6 gm (0.1 mole) of potassium hydroxide in 200 ml of absolute ethanol is added 20.2 gm (0.1 mole) of 2,4-dinitrochlorobenzene. The resultant deep red solution is stirred at room temperature for 1 hr; then enough glacial acetic acid is added dropwise to produce a light yellow solution. The solution is poured into 1.5 liters of cold water. The yellow oil which separates is gradually converted to crystals. The solid ?-butyl JV-(2,4-dinitrophenoxy)carbamate is separated, dried, and recrystallized from an ethyl acetate-hexane mixture to afford 16.4 gm (53%), m.p. 74-75°C. To 15 ml of trifluoroacetic acid is added 4 gm (0.0133 mole) of the i-butyl J/V-(2,4-dinitrophenoxy)carbamate. After the evolution of carbon dioxide has subsided, the solution is poured into 100 ml of ice water. The resultant oily layer crystallizes on standing to afford 2.5 gm (95%), m.p. 112°C (from ethanol).

Recently it was discovered that the alkylation of ethyl JV-hydroxy-carbamate under alkaline conditions, particularly in a DMF medium at 60°C in the presence of sodium bicarbonate, leads to the ultimate formation of O-alkylated hydroxylamines. On the other hand, at 80-85°C, the direct alkylation without the presence of a base ultimately leads to N-alkylhydrox-ylamines (see Table I) [59]. The reaction of ethylazidoformate with an alcohol, while perhaps hazardous, may have some merit (Eqs. 31-33). The overall yield, based on ethyl chloroformate, is said to be on the order of 60%.
 | [Synthesis]
General procedure for the synthesis of 2,4-dinitrophenylhydroxylamine from 2-(2,4-dinitrophenoxy)-isoindole-1,3-dione: first, compound 1-2 2-(2,4-nitrophenoxy)isoindoline-1,3-dione 17.74 g (53.88 mmol) was prepared, and then the hydrazine monohydrate was dissolved in 350 mL of dichloromethane under the protection of argon. 7.6 g (157.33 mmol) of hydrazine monohydrate was added and the reaction was stirred for 4 hours. After completion of the reaction, the reaction mixture was poured into stirred 1N HCl and the resulting solid was filtered. The solid was washed with acetonitrile (MeCN) and the filtrate was extracted with methylene chloride (MC), the organic layers were combined and dried with anhydrous magnesium sulfate (MgSO4) to give 10.50 g of the target compound 1-2 (98% yield). | [References]
[1] Patent: KR101551895, 2015, B1. Location in patent: Paragraph 0171-0172; 0180-0181
[2] Patent: KR2015/74833, 2015, A. Location in patent: Paragraph 0134-0135; 0138-0139; 0191; 0194-0195
[3] Patent: KR2015/74793, 2015, A. Location in patent: Paragraph 0093-0094; 0097-0098
[4] Organic Letters, 2017, vol. 19, # 8, p. 1994 - 1997
[5] Patent: WO2009/146358, 2009, A1. Location in patent: Page/Page column 87-88 |
|
|