| Identification | Back Directory | [Name]
N-[6-(2-hydroxyethoxy)-5-(2-methoxyphenoxy)-2-[2-(2H-tetrazol-5-yl)pyridin-4-yl]pyrimidin-4-yl]-5-methyl-pyridine-2-sulfonamide | [CAS]
180384-56-9 | [Synonyms]
VML 588
Clazosentan
Octahydroaminoacridine succinate
N-(2-(2-(2H-tetrazol-5-yl)pyridin-4-yl)-6-(2-hydroxyethoxy)-5-(2-methoxyphenoxy)pyrimidin-4-yl)-5-methylpyridine-2-sulfonamide
N-[6-(2-HYDROXYETHOXY)-5-(2-METHOXYPHENOXY)-2-[2-(2H-TETRAZOL-5-YL)-4-PYRIDINYL]-4-PYRIMIDINYL]-5-METHYL-2-PYRIDINESULFONAMIDE
N-[6-(2-hydroxyethoxy)-5-(2-methoxyphenoxy)-2-[2-(2H-tetrazol-5-yl)pyridin-4-yl]pyrimidin-4-yl]-5-methyl-pyridine-2-sulfonamide
2-Pyridinesulfonamide, N-[6-(2-hydroxyethoxy)-5-(2-methoxyphenoxy)-2-[2-(2H-tetrazol-5-yl)-4-pyridinyl]-4-pyrimidinyl]-5-methyl- | [Molecular Formula]
C25H23N9O6S | [MOL File]
180384-56-9.mol | [Molecular Weight]
577.57 |
| Chemical Properties | Back Directory | [Melting point ]
239-241° | [Boiling point ]
754.5±70.0 °C(Predicted) | [density ]
1.485±0.06 g/cm3(Predicted) | [storage temp. ]
Store at -20°C | [solubility ]
DMSO : 83.33 mg/mL (144.28 mM; Need ultrasonic) | [form ]
Solid | [pka]
2.97±0.10(Predicted) | [color ]
Off-white to light yellow | [InChIKey]
LFWCJABOXHSRGC-UHFFFAOYSA-N | [SMILES]
C1(S(NC2C(OC3=CC=CC=C3OC)=C(OCCO)N=C(C3C=CN=C(C4=NNN=N4)C=3)N=2)(=O)=O)=NC=C(C)C=C1 |
| Hazard Information | Back Directory | [Description]
Clazosentan (trade name Pivlaz) is a small molecule endothelin (ET) A receptor selective antagonist developed by Idorsia Pharmaceuticals. By inhibiting the ET A receptor, clazosentan sodium can reduce ET-related cerebral vasospasm that may occur after cerebral aneurysmal subarachnoid hemorrhage. | [Uses]
Clazosentan, a novel endothelin A antagonist, improves cerebral blood flow and behavior after traumatic brain injury. | [Synthesis]
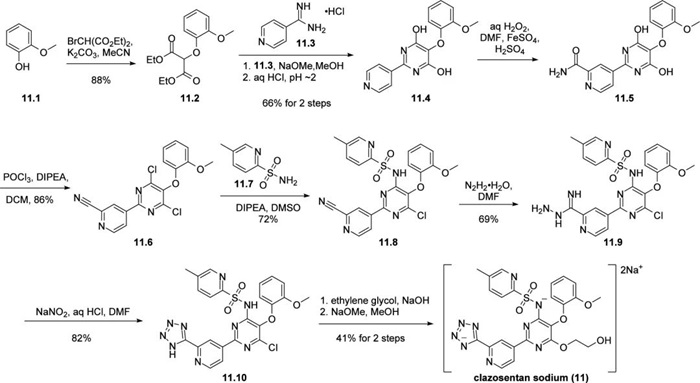
Under alkaline conditions, o-hydroxyanisole (11.1) was alkylated with diethyl bromomalonate, followed by cyclization of the diester 11.2 and the amidine 11.3 to give the pyrimidine 11.4. Amidation of the pyridine ring was performed under modified Minisci conditions, but no yields were reported for the conversion of pyridine 11.4 to 2-carboxyamide pyridine 11.5. Addition of dihydroxypyrimidine 11.5 to phosphorus oxychloride under alkaline conditions resulted in conversion of the hydroxyl group to the corresponding chloride, with simultaneous dehydration of the amide to give the nitrile 11.6. Substitution with commercially available sulfonamide 11.7 under mild conditions allowed the introduction of a single pyridinesulfonamide 11.8, followed by nucleophilic addition of hydrazine to the nitrile to give the iminohydrazine 11.9. Treatment with sodium nitrite under weakly acidic conditions promoted tetrazole formation, followed by substitution of the remaining aryl chloride with ethylene glycol in the presence of sodium hydroxide. The remaining aryl chloride underwent substitution with ethylene glycol. Finally, exposure of clazotan to heated sodium methoxide/methanol followed by careful cooling and filtration afforded the disodium salt of clazotan sodium (11) in 41% yield from chloropyrimidine 11.10.
 | [in vivo]
Clazosentan (10 μM, 0.05?mL/kg, intracisternal injection) inhibits the contractile responses to ET-1 in rats[2].
Clazosentan (10 mg/kg, s.c.) inhibits IL-33-induced hypernociception in mice[4]. | Animal Model: | Rats[2] | | Dosage: | 10 μM, 0.05?mL/kg | | Administration: | Intracisternal injection | | Result: | Inhibited the contractile responses to ET-1, without preventing SAH-induced upregulation of ET receptors in cerebral arteries. |
| [storage]
Store at -20°C |
|
| Company Name: |
MIDECO Gold
|
| Tel: |
023-68625073 15723208759 |
| Website: |
www.midecopharm.com/ |
| Company Name: |
BOC Sciences
|
| Tel: |
|
| Website: |
https://www.bocsci.com |
| Company Name: |
Energy Chemical
|
| Tel: |
021-58432009 400-005-6266 |
| Website: |
http://www.energy-chemical.com |
|