| Identification | Back Directory | [Name]
nifurtimox | [CAS]
23256-30-6 | [Synonyms]
Lampit
BAY-2502
NIFURIMOX
Bayer 2502
Bayer-2502
Aids007325
nifurtimox
BAY-A-2502
Aids-007325
nifurtimox USP/EP/BP
(±)-Nifurtimox Lampit
3-Methyl-N-[(5-nitro-2-furanyl)Methylene]-
LDH,Lactate Dehydrogenase,Parasite,inhibit,Inhibitor,Nifurtimox
4-[(5-Nitrofurfurylidene)amino]-3-methylthiomorpholine 1,1-dioxide
Thiomorpholine, 3-methyl-4-((5-nitrofurfurylidene)amino)-,1,1-dioxide
3-Methyl-N-[(5-nitro-furan-2-yl)Methylene]-4-thioMorpholinaMine1,1-dioxide
3-Methyl-N-[(5-nitro-2-furanyl)methylene]-4-thiomorpholinamine 1,1-dioxide
3-Methyl-4-(5′-nitrofurylidene-amino)-tetrahydro-4H-1,4-thiazine-1,1-dioxide
4-Thiomorpholinamine, 3-methyl-N-[(5-nitro-2-furanyl)methylene]-, 1,1-dioxide
(E)-N-(3-methyl-1,1-dioxo-1,4-thiazinan-4-yl)-1-(5-nitrofuran-2-yl)methanimine
(RS)-3-methyl-N-[(1E)-(5-nitro-2-furyl)methylene]thiomorpholin-4-amine 1,1-dioxide | [EINECS(EC#)]
245-531-0 | [Molecular Formula]
C10H13N3O5S | [MDL Number]
MFCD00869254 | [MOL File]
23256-30-6.mol | [Molecular Weight]
287.295 |
| Chemical Properties | Back Directory | [Melting point ]
177-183°C | [Boiling point ]
550.3±50.0 °C(Predicted) | [density ]
1.4716 (rough estimate) | [refractive index ]
1.6390 (estimate) | [storage temp. ]
Refrigerator | [solubility ]
DMSO: ≥13mg/mL | [form ]
powder | [pka]
-1.01±0.40(Predicted) | [color ]
yellow to orange | [Water Solubility ]
33g/L(temperature not stated) | [BCS Class]
3 | [InChI]
1S/C10H13N3O5S/c1-8-7-19(16,17)5-4-12(8)11-6-9-2-3-10(18-9)13(14)15/h2-3,6,8H,4-5,7H2,1H3/b11-6+ | [InChIKey]
ARFHIAQFJWUCFH-IZZDOVSWSA-N | [SMILES]
CC1CS(=O)(=O)CCN1\N=C\c2ccc(o2)[N+]([O-])=O |
| Hazard Information | Back Directory | [Chemical Properties]
Orange Solid | [Uses]
Antiprotozoal (Trypanosoma) | [Uses]
Antiprotozoal. Showing anti-Trypanosoma cruzi activity. | [Description]
Nifurtimox is manufactured by Bayer and marketed under the trade name Lampits.It is a nitrofuran derivative that was developed specifically for the treatment of American trypanosomiasis (Chagas'disease) (Packachanian, 1957). Nifurtimox is one of two drugs approved for use in treatment of Chagasdisease. It was shown to be the most active and least toxic of this group of agents in preclinical studies and was evaluated in clinical trials in the 1960s and subsequently marketed for use in Chagas’ disease in Latin America in the late 1960s and early 1970s. Although the use of nifurtimox for Chagas’ disease has decreased with the availability of benznidazole, a potentially more active and less toxic agent, there has been a resurgence of interest in and use of nifurtimox for the treatment of second-stage human African trypanosomiasis (HAT)caused by Trypanosoma brucei gambiense. | [Definition]
ChEBI: Nifurtimox is a nitrofuran antibiotic. | [General Description]
Nifurtimox acts as a hypoxia-activated cytotoxin, which specifically kills clonogenic tumor cells under hypoxic conditions. It is used to treat Chagas disease and African trypanosomiasis. Nifurtimox inhibits neuroblastoma and medulloblastoma cell growth. | [Pharmaceutical Applications]
A water-soluble synthetic compound available for oral use. It
exhibits antibacterial activity typical of the group, but its most
notable property is its activity against trypanosomes, especially
Trypanosoma cruzi.
A plasma concentration of 0.5–1 mg/L is achieved c. 2 h
after an oral dose of 15 mg/kg. The plasma half-life is 2–4 h. In
common with other nitrofurans, it is rapidly and extensively
metabolized, so that less than 1% of a dose is excreted intact
in the urine. In renal failure, clearance is somewhat reduced
but the half-life is unchanged.
Adverse events are common. Many patients experience
anorexia, which may be combined with vomiting and abdominal
pain. There may also be neurological reactions such as restlessness,
insomnia, headache and disorientation.
It is used in the treatment of Chagas disease (South
American trypanosomiasis). It has also found some use in the
treatment of African sleeping sickness in combination with
eflornithine. | [Biochem/physiol Actions]
Nifurtimox is a nitrofurane derivative used to treat diseases caused by trypanosomes. Nifurtimox was discovered empirically and its mechanism of action is unclear. It is believed that nifurtimox exerts its biological activity through the bioreduction of the nitro-group to a nitro-anion radical which undergoes redox-cycling with molecular oxygen. | [Mechanism of action]
The drug is given orally and is well absorbed from
the gastrointestinal tract. It is rapidly metabolized, and
only low levels are found in blood and tissues.The drug
is excreted in the urine, primarily in the form of
metabolites. | [Clinical Use]
Nifurtimox is trypanocidal and exerts an effect on
the trypomastigote and amastigote forms of T. cruzi. It
is effective in the treatment of the acute form of
Chagas’ disease but is less effective once the disease becomes
chronic. The drug is moderately well tolerated,
and treatment generally lasts 3 to 4 months. Cure rates
of 80 to 90% have been reported. Since much of the tissue
damage caused by the disease is irreversible, early
diagnosis and treatment are important. Nifurtimox has
also been used in T. gambiense infection with meningoencephalopathic involvement. | [Synthesis]
Nifurtimox, 1,1-dioxide 4-[(5-nitrofuryliden)amino]-3-methylthiomorpholine
(37.4.7), is made by the following scheme. Interaction of 2-mercaptoethanol with propylene
oxide in the presence of potassium hydroxide gives (2-hydroxyethyl)-(2-hydroxypropylsul�fide) (37.4.3), which undergoes intramolecular dehydration using potassium bisulfate to
make 2-methyl-1,4-oxithiane (37.4.4). Oxidation of this using hydrogen peroxide gives
2-methyl-1,4-oxithian-4,4-dioxide (37.4.5), which when reacted with hydrazine transforms
to 4-amino-3-methyltetrahydro-1,4-thiazin-1,1-dioxide (37.4.6). Reacting this with 5-nitro�furfurol gives the corresponding hydrazone?athe desired nifurtimox.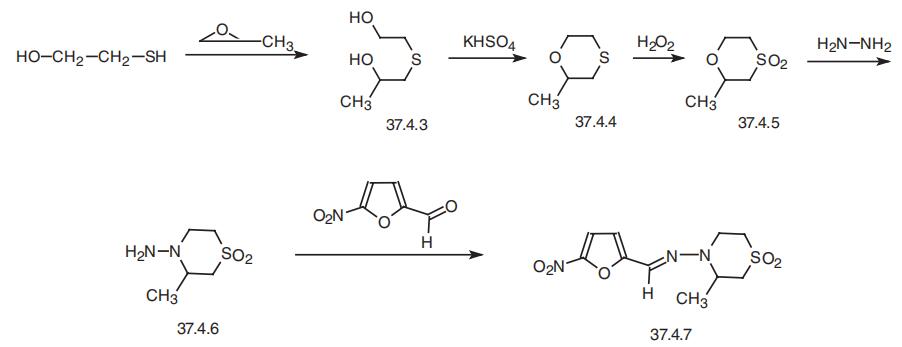
|
| Safety Data | Back Directory | [WGK Germany ]
3 | [Storage Class]
11 - Combustible Solids | [Toxicity]
LD50 in mice, rats (mg/kg): 3720, 4050 by gavage (Hoffmann) |
| Questions And Answer | Back Directory | [Pharmacology and mechanism of action]
Nifurtimox is a nitrofuran derivative that has trypanocidal activity against both the trypomastigote forms (extracellular) and the amastigote forms (intracellular) of Trypanosoma (T.) cruzi. Under experimental conditions amastigotes are 10 times more sensitive to the drug than the trypomastigotes[1]. The mechanism of action of the drug is not clearly known. Its trypanocidal action may be related to its ability to undergo partial reduction to form chemically reactive radicals that cause production of superoxide anion, hydrogen peroxide and hydroxyl radicals. These free radicals react with cellular macromolecules and cause membrane injury, enzyme inactivation, damage to DNA, and mutagenesis [2].
| [Indications]
Treatment of American trypanosomiasis (Chagas’ disease) due to Trypanosoma cruzi. The drug may also be used in patients with Trypanosoma brucei gambiense sleeping sickness who are refractory to other treatments.
| [Side effects]
Side effects of nifurtimox are frequent and can be encountered in up to 40% in children, and up to 70% in adults treated for acute and chronic Chagas’ disease. Common side effects include anorexia, nausea, vomiting, abdominal pain, excitation, sleeping difficulties, dizziness, headache and joint and muscle pains [3]. During treatment, half of the patients may interrupt therapy because of side effects. Other rare side effects include skin eruptions and paraesthesia [4].
| [Contraindications and precautions]
The drug should be given with caution to patients with a history of convulsions, brain injury, peripheral neuropathy and psychiatric illness. Dosage reductions may be considered in patients with liver diseases.
| [Interactions]
Concomitant administration of nifurtimox with melarsoprol[5] or eflornithine[6]have been reported to have synergistic effects in experimental animals (mice) infected with Trypanosoma brucei species. The clinical implication of this is unknown.
| [Preparations]
Lampit (Bayer). Tablets 30 mg, 120 mg.
| [References]
1. Webster LT Jr (1990). Drugs used in the chemotherapy of profozoal infections. In: Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 8th edn, edited by A.G.Gilman, T.W.Rall, A.S.Nies, P.Taylor, (New York: Pergamon Press), pp. 1010–1011.
2. Docampo R, Moreno SNJ, Stoppani AOM, Leon W, Cruz FS, Villalta F, Muniz RFA (1981). Mechanism of nifurtimox toxicity in different forms of Trypanosoma cruzi. Biochem Pharmacol, 30, 1947–1981.
3. Gutteridge WE (1985). Existing chemotherapy and its limitations. Br Med Bull, 41, 162–168.
4. Wegner DHG, Rohwedder RW (1972). The effects of nifurtimox in acute Chagas’ infection. Arzneimittelforschung, 22, 1624–1635.
5. Jennings FW (1991). Chemotherapy of CNS-trypanosomiasis: the combined use of the arsenicals and nitro-compounds. Trop Med Parasitol, 42, 139–142.
6. Jennings FW (1988). The potentiation of arsenicals with difluoromethylornithine (DFMO): experimental studies in murine trypanosomiasis. Bull Soc Pathol Exot, 81, 595–607.
|
|
|