| Identification | Back Directory | [Name]
Vitexin | [CAS]
3681-93-4 | [Synonyms]
C01460
VITEXIN
Vitxein
Vitexina
VITEXIN(P)
VITEXIN(SH)
ORIENTOSIDE
VITEXIN 98+%
VITEXIN(P)(CALL)
VITEXIN WITH HPLC
8-Glucosylapigenin
Apigenin 8-glucoside
Isovitexin(C-8 isoMer)
APIGENIN-8-C-GLUCOSIDE
Apigenin-8-O-glucoside
8-Glucopyranosylapigenin
APIGENIN-8-C-GLUCOSIDE hplc
8-C-β-D-Glucopyranosylapigenin
8-beta-d-glucopyranosyl-apigenin
Vitexin, froM Crateagus pinnatifida
8-d-glucosyl-4’,5,7-trihydroxy-flavon
Flavone,8-D-glucosyl-4',5,7-trihydroxy-
4H-1-Benzopyran-4-one,8-β-D-glucopyranosyl-5,7-dihydroxy-2-(4-hydroxyphenyl)
4H-1-Benzopyran-4-one,8-b-D-glucopyranosyl-5,7-dihydroxy-2-(4-hydroxyphenyl)-
2-(4-Hydroxyphenyl)-5,7-dihydroxy-8-(β-D-glucopyranosyl)-4H-1-benzopyran-4-one
8-(β-D-Glucopyranosyl)-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one
4h-1-benzopyran-4-one,5,7-dihydroxy-8-beta-d-glucopyranosyl-2-(4-hydroxyphenyl
8-beta-D-glucopyranosyl-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one
5,7-Dihydroxy-8-beta-D-glucopyranosyl-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one
Vitexin
5,7-Dihydroxy-8-beta-D-glucopyranosyl-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one
5,7-Dihydroxy-2-(4-hydroxyphenyl)-8-[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]chromen-4-one | [EINECS(EC#)]
222-963-8 | [Molecular Formula]
C21H20O10 | [MDL Number]
MFCD00017456 | [MOL File]
3681-93-4.mol | [Molecular Weight]
432.38 |
| Chemical Properties | Back Directory | [Melting point ]
256-257°C | [Boiling point ]
767.7±60.0 °C(Predicted) | [density ]
1.686±0.06 g/cm3(Predicted) | [storage temp. ]
Inert atmosphere,Room Temperature | [solubility ]
DMSO (Slightly), Pyridine (Slightly) | [form ]
neat | [pka]
6.27±0.40(Predicted) | [color ]
Yellow | [BRN ]
67796 | [Stability:]
Hygroscopic | [Major Application]
food and beverages | [Cosmetics Ingredients Functions]
SKIN PROTECTING
ANTIOXIDANT
SKIN CONDITIONING - HUMECTANT
HUMECTANT | [InChIKey]
SGEWCQFRYRRZDC-VPRICQMDSA-N | [SMILES]
C12OC(=CC(=O)C=1C(=CC(O)=C2[C@H]1[C@H](O)[C@H]([C@H](O)[C@@H](CO)O1)O)O)C1=CC=C(O)C=C1 |&1:12,13,15,16,18,r| | [LogP]
-0.014 (est) |
| Hazard Information | Back Directory | [Chemical Properties]
Yellow powder | [Uses]
Vitexin is a phenolic glycoside drug that shows anti-stress activity. It also shows possible application as an anti-diabetic. | [Definition]
ChEBI: An apigenin flavone glycoside, which is found in the passion flower, bamboo leaves and pearl millet | [Hazard]
poison | [storage]
Room temperature |
| Questions And Answer | Back Directory | [Plant sources]
Vitexin, also known as the vitex glycoside, is a natural flavonoid glycoside extracted from leaves of vitex, a plant in the family Verbenaceae. Vitexin is widely distributed in the leaves and stems of dozens of plants in nature, such as vitex, fructus viticis, puberulous glochidion, hawthorn, Ficus microcarpa, Lygodium japonicum, Stenoloma chusanum, Alsophila spinulosa leaf, paper mulberry leaf, and indigowoad leaf, among which the main sources are hawthorn and leaves and stems of plants in the genus Vitex of the family Verbenaceae. Vitexin has a variety of physiological activity, such as anti-cancer, anti-tumor, anti-inflammation, spasmolysis, depressurization, promoting blood circulation and dissipating blood stasis, and regulating qi-flowing and invigorating pulse-beat. Clinically, vitexin mainly used for the treatment of cardiovascular diseases, for example, for the treatment of thoracic obstruction caused by blood stasis congesting veins. The syndrome includes chest tightness, suffocation, precordial tingling, heart palpitations, forgetfulness, dizziness, tinnitus, angina pectoris, hyperlipidemia, insufficient blood supply to the heart, and so on. In addition, this product can also enhance the adrenal function and increase the phagocytic activity of monocyte-macrophage system. | [Physical and chemical properties]
Yellow powder; melting point, 258-259℃ (265℃); optical rotation, [α] D18 (c = 2, pyridine). | [Chemical constituents]
The vitex leaf contains volatile oil, of which the main constituent is β-caryophyllene, followed by the sabinene. It also contains α-thujene, α-pinene, β-pinene, camphene, α-phellandrene, p-cymene, limonene, 1, 8-cineole as well as some stomachic ingredients with bitter taste such as vitexilactone, agnuside, ,artemisetin, and p-hydroxybenzoic acid. | [Determination]
TLCS method
(1) Chromatographic condition: RP-18F254s efficient reversed-phase TLC plate was used. Impregnate the plate in 70% tetrahydrofuran-water solution (containing 0.1% tetrabutylammonium bromide) for 10 minutes and dried. Tetrahydrofuran-water (46:54, containing 0.1% tetrabutylammonium bromide) was used as developing solvent. The total distance traveled by the mobile phase was 4.5 cm; detected under UV light (254 nm).
(2) Prepare reference solution: weigh proper amount of vitexin and p-hydroxybenzoic acid accurately, and add anhydrous ethanol to prepare 0.1 mg/ml vitexin and 0.05 mg/ml p-hydroxybenzoic acid solution as reference solution.
(3) Prepare sample solution: weigh accurately 5 g of fructus viticis powder (particles were passing through a 40-mesh sieve and were dried to constant weight) and soak with anhydrous ethanol for eight hours in a Soxhlet extractor. Recover the solvent to a 25 ml volumetric flask and dilute with anhydrous ethanol to volume. Take the final solution as sample solution.
(4) Determination: Imbibe accurately 2 μl of each of the sample solutions and the reference solution, and spot onto the same TLC plate, developing under the above chromatographic condition, then take out the plate and dry. The samples were then analyzed according to the TLC-scanning method and the single wavelength reflection sawtooth scanning method. The detection wavelength 350 nm and 258 nm was used respectively for quantification of vitexin and p-hydroxybenzoic acid. The SX value was 7, and the slit dimension was kept at 6 mm × 0.2 mm. The integral value of light absorbance of the samples and the reference were determined and the sample contents were calculated using the external standard method.
(5) The chromatogram
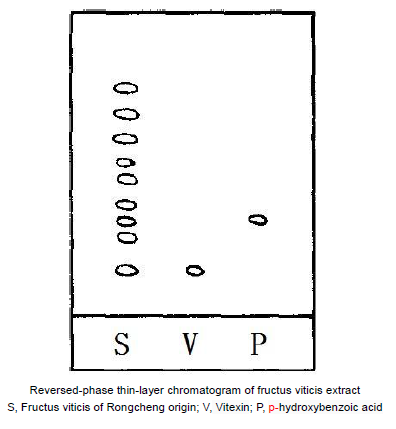
Figure 1 is a reversed-phase thin-layer chromatogram of fructus viticis extract. S, Fructus viticis of Rongcheng origin; V, Vitexin; P, p-hydroxybenzoic acid
(6) Determination results
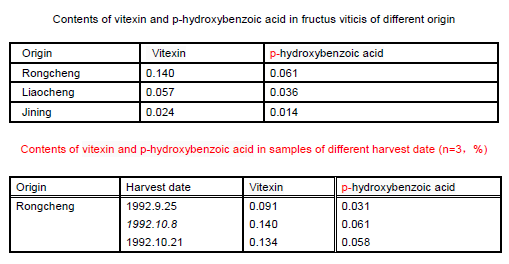
Figure 2 shows the contents of vitexin and p-hydroxybenzoic acid in samples of different origin or harvest date (n=3,%) | [Pharmacological effect]
The volatile oil contained in the vitex leaf has significant expectorant effect and play a role in relieving cough by inhibiting the cough center. It also has anti-histamine effect, can relieve bronchial smooth muscle spasm, and thus relieves asthma. Emulsion of the vitex leaf oil has a significant and long-lasting antihypertensive effect on rabbit blood pressure. Leaf oil of vitex can improve the phagocytic capacity of macrophages, promote the serum protein synthesis and regulate the function of immunoglobulin, and has certain sedative and hypnotic effects when taken orally. The decoction made by the stems or leaves of vitex has significant antibacterial effect against Staphylococcus aureus and Bacillus anthracis, and it also has inhibitive effect against the E. coli, beta streptococcus, Corynebacterium diphtheriae, typhoid bacillus, Pseudomonas aeruginosa and Shigella.
Vitexin has an obvious protective effect on acute myocardial ischemia of rats. The possible mechanism may be due to the activation of antioxidant enzymes which enhances the activity of SOD and GSH-Px, and reduce the tissue damage by excessive oxygen free radicals. In the meanwhile, it may function against the calcium overload by preventing the Ca2+ influx in a Ca2+ antagonist-like meaner, increasing the Ca2+ uptake of sarcoplasmic reticulum and by reducing the concentration of intracellular free Ca2+, which prevents the myocardial cell from further injury caused by Ca2+ overload and reduces the damage in the endothelial cell, and thus reduces the damage of biomembranes, ion pumps and the entire cardiac cells and ultimately shows the protective effect on acute myocardial ischemia. However, the starting and links of its roles needs further study. In summary, Vitexin has a good protective effect on ischemic myocardial injury. The role may relate to reducing the size of ischemic myocardial infarct, decreasing the viscosity of plasma and whole blood, increasing RBC deformability, and inhibiting thrombosis. | [Efficacy and application]
1. Vitexin Used for treatments of colds and cough. It also can be used with the perilla leaf in cure of common cold due to wind-cold. It has been used presently to cure chronic bronchitis, with good clinical result.
2. Used for treatments of abdominal pain and vomiting and diarrhea due to summer-heat and damp. It also can be used with herba centellae to cure vomiting and diarrhea due to summer-heat and damp.
3. Used for treatments of itching caused by rubella, athlete's foot, athlete's foot swelling, mastitis, insect or snake bites. It is usually smashed into small pieces and covered onto the affected area, or boiled with water and use the decoction to wash the affected area. It also can be used with loofah, perilla, drug sweetflag rhizome or folium artemisiae argyi to cure athlete's foot swelling. |
|
|