| Identification | Back Directory | [Name]
2,6-Difluoro-3-nitropyridine | [CAS]
58602-02-1 | [Synonyms]
2,6-Difluor-3-nitropyridin
6-difluoro-3-nitropyridine
2,6-Difluoro-5-nitropyridine
3-Nitro-2,6-difluoropyridine
2,6-Difluoro-3-nitropyridine
PYRIDINE, ,26-DIFLUORO-3-NITRO- | [Molecular Formula]
C5H2F2N2O2 | [MDL Number]
MFCD11044323 | [MOL File]
58602-02-1.mol | [Molecular Weight]
160.08 |
| Chemical Properties | Back Directory | [Melting point ]
6℃ | [Boiling point ]
218-220℃ (760 Torr) | [density ]
1.555±0.06 g/cm3 (20 ºC 760 Torr) | [Fp ]
110.4±25.9℃ | [storage temp. ]
Inert atmosphere,2-8°C | [pka]
-10.13±0.10(Predicted) | [Appearance]
Colorless to light yellow Liquid |
| Questions And Answer | Back Directory | [Preparation]
Interestingly, direct nitration of 2-fluoro and 2,6-difluoropyridine with nitronium tetrafluoroborate achieved a degree of success and whilst 2-fluoropyridine gave mostly the N-nitro salt, a 6% yield of 5-nitro-2-fluoropyridine was obtained and 2,6-difluoropyridine gave a 20% yield of 3-nitro-2,6-difluoropyridine.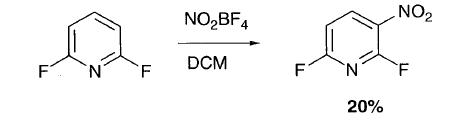 |
| Hazard Information | Back Directory | [Synthesis]
The general procedure for the synthesis of 2,6-difluoro-3-nitropyridine from 2,6-dichloro-3-nitropyridine was as follows: 3-bromo-2,6-dichloropyridine (4.70 g, 20.7 mmol) was dissolved in DMSO (103 mL) at room temperature, followed by the addition of cesium fluoride (12.6 g, 82.9 mmol). The reaction mixture was stirred at 80°C for 8 hours in air. After completion of the reaction, the mixture was poured into water and extracted with ether (Et2O). The organic layer was separated, washed sequentially with water and brine, dried over anhydrous sodium sulfate (Na2SO4) and subsequently concentrated under reduced pressure (400 Torr, 40°C). The residue was purified by silica gel column chromatography using ethyl acetate (EtOAc) solution in hexane as eluent to afford 3-bromo-2,6-difluoropyridine (3B) as a colorless oil (2.58 g, 64% yield). Its nuclear magnetic resonance hydrogen spectrum (1H NMR, CDCl3) data were as follows: δ 6.79 (1H, dd, J = 8.3, 3.0 Hz), 8.03 (1H, ddd, J = 8.4, 8.4, 7.0 Hz). Nuclear Magnetic Resonance Fluorine Spectroscopy (19F NMR, CDCl3) data: δ -69.3 Hz, -63.8 Hz. compounds 4B to 8B were prepared similarly as described for 3B. | [References]
[1] Collection of Czechoslovak Chemical Communications, 1991, vol. 56, # 11.1, p. 2420 - 2429
[2] Tetrahedron Letters, 2015, vol. 56, # 44, p. 6043 - 6046 |
|
| Company Name: |
T&W GROUP
|
| Tel: |
021-61551611 13296011611 |
| Website: |
www.trustwe.com/ |
|