| Identification | Back Directory | [Name]
Beryllium hydride | [CAS]
7787-52-2 | [Synonyms]
Beryllium hydride.
beryllium dihydride
Dihydrogen beryllium salt
beryllium(+2) cation: hydrogen(-1) anion | [Molecular Formula]
BeH2 | [MOL File]
7787-52-2.mol | [Molecular Weight]
11.03 |
| Chemical Properties | Back Directory | [density ]
0.650 | [solubility ]
reacts with H2O; insoluble in ethyl ether, toluene | [form ]
white amorphous solid | [color ]
White | [Water Solubility ]
reacts slowly with H2O, rapidly with dilute acids evolving H2(g) [MER06] |
| Hazard Information | Back Directory | [Chemical Properties]
white, amorphous solid; inert to laboratory air; can be prepared by continuous thermal decomposition of a di-t-butylberyllium ethyl ether complex in a boiling hydrocarbon; has found use as rocket fuel and as a moderator for nuclear reactors [KIR80] [MER06] | [Description]
BeH2 was first synthesized in 1951 by reacting dimethylberyllium,
Be(CH3)2, with lithium aluminum
hydride, LiAlH4. A purer grade of BeH2 can also be
formed from the pyrolysis of di-tert-butylberyllium,
{Be(C(CH3)3)2} at 210°C. The purest beryllium hydride
is obtained by the reaction of triphenylphosphine, i.e.
PPh3,with berylliumborohydride,Be(BH4)2 by the reaction:
Be(BH4)2+2PPh3→2Ph3PBH3 + BeH2
Note that unlike the other elements in Group IIA
where the hydride can be prepared by reaction of the
elements, the reaction of the metal with hydrogen to
produce beryllium hydride has not proved possible.
BeH2 is usually formed as an amorphous white solid,
but a hexagonal crystalline form with a higher density
(~0.78 g/cm3) has also been reported. This was prepared
by heating amorphous BeH2 under pressure, with
0.5–2.5% LiH as a catalyst. A more recent investigation
found that crystalline beryllium hydride has a bodycentered
orthorhombic unit cell, containing a network
of corner-sharing BeH4 tetrahedra (there are 12BeH2
molecules in the unit cell) in contrast to the flat,
hydrogen-bridged, infinite chains previously thought
to exist in crystalline BeH2. Studies of the amorphous
form also find that it consists of a network of cornershared
tetrahedra. The density is 0.755 g/cm3. | [Physical properties]
White amorphous solid; density 0.65 g/cm3; decomposes at 250°C; reacts with water. | [Uses]
Beryllium hydride (BeH2) liberates hydrogen gas when mixed with water. It is used as a
source of hydrogen in experimental rockets and fuel cells. | [Definition]
ChEBI: Beryllium dihydride is a beryllium hydride. | [Preparation]
Beryllium hydride is made by treating an ethereal solution of beryllium borohydride with triphenylphosphine, or by pyrolysis of di-tert-butylberyllium. | [Safety Profile]
Confirmed carcinogen. Adangerous fire hazard. When heated to 220°C it liberatesexplosive hydrogen gas. Reacts violently with methanol,water, and dilute acids. When heated to decomposition itemits toxic fumes of BeO. See BERYLLIUMCOMPOUNDS and HYDRIDE |
| Questions And Answer | Back Directory | [Chemical Properties]
Beryllium hydride (BeH2) as a solid and also as a molecular polymer (upto decamer). It does not have any common uses. All beryllium compounds are quite toxic.
Beryllium hydride (BeH2) is a simple light weight metal hydride well known for its hydrogen storage capability. The crystalline BeH2 has been reported as a three dimensional array of tetrahedral BeH4 molecules and not as BeH2 chains. The beryllium hydride oligomers are obtained from the excited beryllium (1Po) atom and hydrogen molecule. These are very difficult to synthesize and are easily hydrolyzed by dilute acids. Because of its high hydrogen storage capacity (18.2 wt%), it is of considerable interest especially for use in nuclear reactor and also as a rocket fuel.
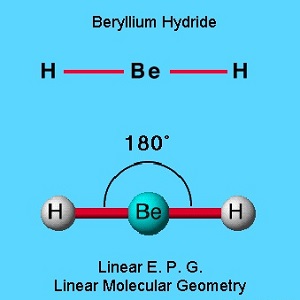
Beryllium hydride is an example of linear electron pair and molecular geometry. This molecule is electron deficient and does not follow the octet rule because it has only 4 valence electrons. The hydrogen atoms are as far apart as possible if opposite each other at 180o. This is linear geometry.
| [Beryllium Compounds]
In the mid-1960s, considerable interest in beryllium hydride was generated by its potential use with ammonium perchlorate oxidizer in solid propellants for rockets. Beryllium hydride, BeH2 , was prepared by action of ethereal lithium-aluminum hydride with beryllium chloride. A white powder resulted from which the complete removal of ether was difficult. The hydride is not stable above 125 °C (255 °F), decomposing into beryllium and hydrogen.
Beryllium hydride is a three-dimensional polymer, considerably ionic in character and containing Be2+ and H- ions. Alternatively, it may be considered to be an electron-deficient molecule, similar to beryllium chloride and the beryllium alkyls, with hydrogen bridges between the two beryllium atoms (BeH2Be).
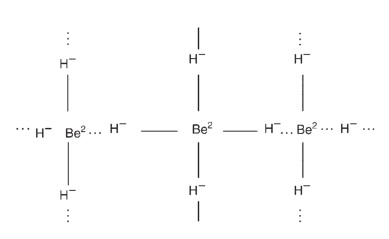
Three-dimensional molecule of beryllium hydride
|
|
|