| Identification | Back Directory | [Name]
ETHOTOIN | [CAS]
86-35-1 | [Synonyms]
AC-695
Accenon
Peganone
Etbotoin
ETHOTOIN
Pegoanone
ETHOTOIN (200 MG)
ETHOTOIN USP/EP/BP
Ethotoin ((±)-Ethotoin)
3-ethyl-5-phenyl-hydantoi
3-Ethyl-5-Phenylhydantoin
Hydantoin, 3-ethyl-5-phenyl-
3-Ethyl-5-phenylimidazolidin-2,4-dione
1-Ethyl-2,5-dioxo-4-phenylimidazolidine
3-ethyl-5-phenylimidazolidine-2,4-dione
3-Ethyl-5-phenyl-2,4-imidazolidinedione
3-ethyl-5-phenyl-imidazolidine-2,4-dione
2,4-Imidazolidinedione, 3-ethyl-5-phenyl-
Hydantoin, 3-ethyl-5-phenyl- (6CI, 7CI, 8CI) | [EINECS(EC#)]
201-665-1 | [Molecular Formula]
C11H12N2O2 | [MOL File]
86-35-1.mol | [Molecular Weight]
204.23 |
| Chemical Properties | Back Directory | [Melting point ]
94°C | [Boiling point ]
342.72°C (rough estimate) | [density ]
1.1754 (rough estimate) | [refractive index ]
1.5200 (estimate) | [storage temp. ]
Refrigerator | [solubility ]
Dichloromethane (Slightly), DMSO (Slightly), Methanol (Slightly) | [form ]
Solid | [pka]
8.01±0.70(Predicted) | [color ]
White to Off-White |
| Hazard Information | Back Directory | [Originator]
Peganone,Abbott,US,1957 | [Uses]
Ethotoin is a hydantoin based anticonvulsant drug used in the treatment of epilepsy. | [Uses]
Ethotoin is less active and less toxic than phenytoin. It is used for the same indications as
is phenytoin, i.e. for control of major and complex epileptic attacks. | [Definition]
ChEBI: An imidazolidine-2,4-dione that is hydantoin substituted by ethyl and phenyl at positions 3 and 5, respectively. An antiepileptic, it is less toxic than phenytoin but also less effective. | [Manufacturing Process]
Benzaldehyde cyanohydrin is reacted with urea to displace the hydroxyl group
of the cyanohydrin. That intermediate is treated with HCl to convert the urea
nitrogen to a nitrile. The resultant imine is hydrolyzed to the phenylhydantoin.
Alkylation with ethyl iodide gives ethotoin, as described by A. Pinner in Chem.
Ber. 21, 2325 (1888). | [Brand name]
Peganone (Ovation. | [Therapeutic Function]
Anticonvulsant | [Synthesis]
Ethotoin, 3-ethyl-5-phenylimidazolidine-2,4-dione (9.1.5), is synthesized in
basically the same manner as described above, which in this case involves the reaction of
benzaldehyde oxynitrile (9.1.2), with urea or ammonium hydrocarbonate, which forms
the intermediate urea derivative (9.1.3) which on acidic conditions (9.1.3) cyclizes to
5-phenylhydantoin (9.1.4). Alkylation of this product using ethyliodide leads to the for�mation of ethotoin (9.1.5) [3,4].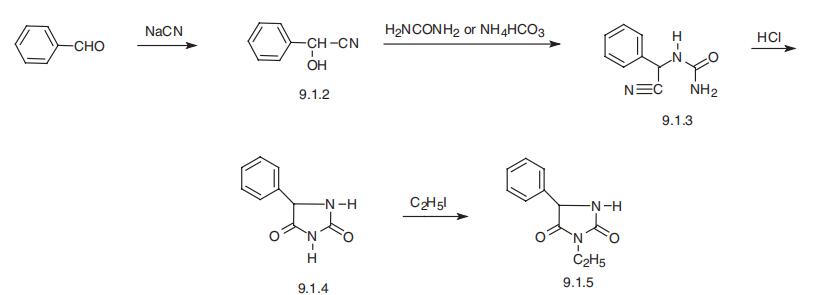
| [Metabolism]
Ethotoin differs from phenytoin in that one phenyl substituent at position 5 has been replaced by hydrogen, and the N-H at
position 3 is replaced by an ethyl group. It may be indicated for treatment of tonic-clonic and complex partial
(psychomotor) seizures. Because it is considered to be less toxic but also less effective and more sedating than phenytoin,
ethotoin usually is reserved for use as an add-on drug. Ethotoin does not share phenytoin's profile of antiarrhythmic
action. The metabolism of ethotoin, like phenytoin, is saturable and nonlinear. Its administration is contraindicated in patients
with hepatic abnormalities and hematologic disorders. | [storage]
Store at 2-8°C, protect from light | [Purification Methods]
It is an anticonvulsant and is used in epilepsy. [Dudley et al. J Heterocycl Chem 10 173 1973, Pinner Chem Ber 21 2320 1888, Beilstein 25 III/IV 963, 27 II 860.] |
|
| Company Name: |
BOC Sciences
|
| Tel: |
|
| Website: |
https://www.bocsci.com |
|