| Identification | Back Directory | [Name]
DPDPE | [CAS]
88373-73-3 | [Synonyms]
DPDPE
enkephalin
Cyclic DPDPE
Enkephalin USP/EP/BP
[D-PEN2,5]-ENKEPHALIN
Y-(D-PEN)-G-F-(D-PEN)
TYR-D-PEN-GLY-PHE-D-PEN
(D-PEN2,D-PEN5)-ENKEPHALIN
M.W. 645.79 C30H39N5O7S2
H-TYR-D-PEN-GLY-PHE-D-PEN-OH
[D-Pen2,5]-Enkephalin hydrate
L-Tyr-D-Pen-Gly-L-Phe-D-Pen-OH
d-penicillamine(2,5)-enkephali
Cyclic [D-Pen2,D-Pen5]enkephalin
Bis(metaboric acid)magnesium salt
Enkephalin, D-Penicillamine (2,5)-
L-Tyr-D-Pen(1)-Gly-L-Phe-D-Pen(1)-OH
[d-pen2,5]-enkephalin acetate hydrate
H-Tyr-D-Pen-Gly-Phe-D-Pen-OH (Disulfide bond)
H-TYR-D-PEN-GLY-PHE-D-PEN-OH (DISULFIDE BRIDGE: 2-5)
(1S,6S,12S)-6-[[(2S)-2-amino-3-(4-hydroxyphenyl)propanoyl]amino]-12-(benzyl)-7,10,13-triketo-2,2,5,5-tetramethyl-3,4-dithia-8,11,14-triazacyclotetradecane-1-carboxylic acid
(1S,6S,12S)-6-[[(2S)-2-amino-3-(4-hydroxyphenyl)propanoyl]amino]-2,2,5,5-tetramethyl-7,10,13-trioxo-12-(phenylmethyl)-3,4-dithia-8,11,14-triazacyclotetradecane-1-carboxylic acid
(1S,6S,12S)-6-[[(2S)-2-azanyl-3-(4-hydroxyphenyl)propanoyl]amino]-2,2,5,5-tetramethyl-7,10,13-trioxo-12-(phenylmethyl)-3,4-dithia-8,11,14-triazacyclotetradecane-1-carboxylic acid | [Molecular Formula]
C30H39N5O7S2 | [MDL Number]
MFCD00076430 | [MOL File]
88373-73-3.mol | [Molecular Weight]
645.79 |
| Questions And Answer | Back Directory | [Discovery]
Endogenous morphines first isolated from the brain,
enkephalin pentapeptides are associated with nociception
by analgesic functions. There are two types of ENK peptides: methionine ENK
(Met-ENK, [Met5]-ENK) and leucine ENK (Leu-ENK,
[Leu5]-ENK). The isolation of these peptides from the
porcine brain was first reported in 1975. | [Structure]
The amino acid sequences of Met-ENK (Tyr-Gly-Gly-Phe-Met) and Leu-ENK (Tyr-Gly-Gly-Phe-Leu) are
common in mammals. These are generated from the common precursor proenkephalin (PENK or, alternatively,
proenkephalin-A [PENK-A]) by proteolytic cleavage. In addition to Met-ENK and Leu-ENK, several distinct
PENK-derived peptides with C-terminal or N-terminal
extensions are present in the bovine brain.
In mammals, including humans, PENK contains six copies of Met-ENK and one copy of Leu-ENK,
whereas in amphibians, lungfish, and sharks, PENK contains seven Met-ENK sequences. In contrast, zebrafish
PENK contains five copies of Met-ENK and one copy
of Leu-ENK.
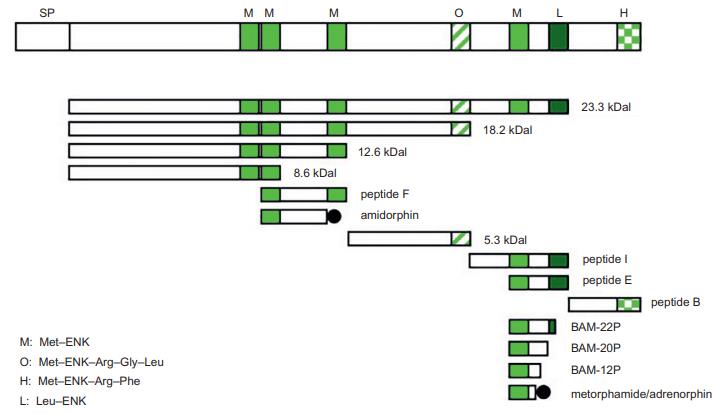 | [Gene, mRNA, and precursor]
The human PENK gene, PENK, location 8q23-q24, consists of three exons and has transcription elements such as
ENKCRE-1 and ENKCRE-2. The latter acts as an
enhancer. A glucocorticoid response element is also pre�sent. Human prePENK is composed of 267 aa residues.
A signal peptide consisting of 24 aa residues is followed
by a cysteine-containing N-terminal sequence and the
region containing the repeated ENK sequences. | [Receptors]
Met-ENK and Leu-ENK are agonists for the δ-opioid
receptor (DOR, also known as δ receptor, DOR-1, OP1,
etc.) or μ-opioid receptor (MOR; also known as μ receptor,
MOR-1, OP3, etc.), both of which are the subtypes of opioid receptors that belong to the GPCR family. Human DOR is located on chromosome 1 (1p36.1-p34.3). MetENK also interacts with a nonclassical opioid receptor
called the opioid growth factor receptor. | [Clinical implications]
Although MOR agonists are the most commonly used
drugs for the treatment of pain, mu agonists show variable efficacy in the treatment of chronic pain, partly
because of the development of tolerance. Delta-opioid
agonists have been shown to have beneficial effects in
chronic pain and emotional disorders, and may potentially be used for treatment in these symptoms.Acute
alcohol intoxication stimulates the release of the endogenous opioid peptides β-endorphin, enkephalin, and
dynorphin, and nonselective antagonists for opioid
receptors reduce alcohol consumption in humans as well
as alcohol consumption and self-administration in rats.
Selective antagonists of MOR and DOR have been shown
to reduce alcohol self-administration. Thus, MOR and
DOR are viable targets for reducing the positive reinforcing effects of alcohol in nondependent cohorts. | [Agonists and Antagonists]
DSLET, diprenorphine, DADLE, (-)-bremazocine,
DPDPE, nalmefene, hydromorphone, and morphine are
agonists. Naltriben, naltrindole, naltrexone, quadazocine, alvimopan, and naloxone are antagonists. | [Biological functions]
The effects of morphine represent the functions of
endogenous opioid peptides via DOR, KOR, and MOR.
In the central nervous system, morphine causes analgesia, euphoria, sedation, miosis (constriction of the pupils),
truncal rigidity, nausea, and vomiting, and decreases the
rate of respiration and the cough reflex. In the gastrointestinal system, morphine causes constipation, constriction
of the biliary smooth muscle, and esophageal reflux,
and reduces gastric motility, digestion in the small intestine, and peristaltic waves in the colon. In other smooth
muscles, morphine causes urinary retention, depresses
renal function, and decreases uterine tone. In the skin,
morphine causes itching, sweating, and flushing of the
face, neck, and thorax. In the cardiovascular system, morphine decreases blood pressure and the heart rate if the
cardiovascular system is stressed. In the immune system,
morphine decreases the cytotoxic activity of natural killer
cells and the formation of rosettes by human lymphocytes. Morphine also induces behavioral restlessness.
Pharmacological studies using both delta agonists and
delta antagonists in rodents show that the anxiolytic
activity of the opioid tone is mediated by DOR. Although
Met-ENK was originally identified as a neuromodulator
that interacts with DOR, this peptide was subsequently
revealed to be a tonically active regulator of cell proliferation as well. | [Synthesis and release]
In the rat, glucocorticoid is required for the maintenance of basal Penk mRNA expression in the forebrain,
and insulin injection induces an increase in mRNA levels
in the adrenal medulla. In humans, as in rats, Met-ENK
that is probably derived from the adrenal medulla is present in the circulation. Acupuncture relief of chronic pain
syndrome correlates with increased plasma Met-ENK
concentration, whereas the level of plasma Met-ENK in
chronic alcoholics is reduced. |
| Chemical Properties | Back Directory | [Boiling point ]
1038.6±65.0 °C(predicted) | [density ]
1.38±0.1 g/cm3(Temp: 20 °C; Press: 760 Torr)(predicted) | [RTECS ]
YV9461100 | [storage temp. ]
−20°C
| [solubility ]
Water:10.0(Max Conc. mg/mL);0.15(Max Conc. mM) | [form ]
Solid | [pka]
2.97±0.70(predicted) | [color ]
White to off-white | [Water Solubility ]
Soluble to 1 mg/ml in water | [Sequence]
Tyr-{Pen}-Gly-Phe-{Pen} (Disulfide bridge:Pen2-Pen5) | [InChIKey]
MCMMCRYPQBNCPH-WMIMKTLMSA-N | [SMILES]
S1SC([C@H](C(=O)NCC(=O)N[C@H](C(=O)N[C@H](C1(C)C)C(=O)O)Cc3ccccc3)NC(=O)[C@@H](N)Cc2ccc(cc2)O)(C)C |
| Hazard Information | Back Directory | [Uses]
[D-Pen2,5]-Enkephalin hydrate (DPDPE) has been used to study the pharmacodynamic and pharmacokinetic capabilities of polyethylene glycol (PEG) conjugate of DPDPE in rodents. It has been used to study the tendency of opiate self-administration in male Long-Evans rats, in ventral tegmental area (VTA). | [Definition]
ChEBI: A heterodetic cyclic peptide that is a cyclic enkephalin analogue, having D-penicillaminyl residues located at positions 2 and 5, which form the heterocycle via a disulfide bond. | [General Description]
[D-Pen2,5]-Enkephalin hydrate, also called DPDPE, is a peptide and acts as a δ1-opioid receptor agonist. Opioid receptors are divided into three types called, μ, κ and δ, depending upon their ligands. These ligands are peptides and are classified as enkephalins, endorphins and dynorphins. | [Biochem/physiol Actions]
Antinociceptive activity mediated through the δ1 receptor while the modulatory activity is mediated through the δ2 receptor. Tritiated [D-Pen2,5]-enkephalin is used as a δ1 ligand. | [Clinical Use]
Nalmefene reduces alcohol consumption in adults
with alcohol dependence. Morphine and hydromorphone are used in the treatment and management of
severe pain. Naltrexone is used alongside behavioral
therapy both for opiate addiction and for alcohol dependency. Naloxone is used to reverse narcotic depression. | [storage]
Store at -20°C |
|
| Company Name: |
Sigma-Aldrich
|
| Tel: |
021-61415566 800-8193336 |
| Website: |
https://www.sigmaaldrich.cn |
|