| Identification | Back Directory | [Name]
Lobeline | [CAS]
90-69-7 | [Synonyms]
C07475
Lobelin
Lobeline
Inflatine
LOBELIDINE95%,98%
Lobeline USP/EP/BP
LOBELINE HCl, a-(P)
L-lobeline free base
Lobeline (base and/or unspecified salts)
2-(6-[2-Hydroxy-2-phenylethyl]-1-methyl-2-piperidinyl)-1-phenylethanone
(-)-α-[(2R,6S)-6-[(S)-β-hydroxyphenethyl]-1-methyl-2-piperidinyl]acetophenone
Ethanone, 2-[(2R,6S)-6-[(2S)-2-hydroxy-2-phenylethyl]-1-methyl-2-piperidinyl]-1-phenyl- | [EINECS(EC#)]
202-012-3 | [Molecular Formula]
C22H27NO2 | [MDL Number]
MFCD00056052 | [MOL File]
90-69-7.mol | [Molecular Weight]
337.455 |
| Chemical Properties | Back Directory | [Melting point ]
130-131° | [alpha ]
D15 -43° (alc) | [Boiling point ]
473.76°C (rough estimate) | [density ]
1.0909 (rough estimate) | [refractive index ]
1.5614 (estimate) | [pka]
14.34±0.20(Predicted) | [Optical Rotation]
-4315 (c1, ethanol) | [InChI]
InChI=1S/C22H27NO2/c1-23-19(15-21(24)17-9-4-2-5-10-17)13-8-14-20(23)16-22(25)18-11-6-3-7-12-18/h2-7,9-12,19-21,24H,8,13-16H2,1H3/t19-,20+,21-/m0/s1 | [InChIKey]
MXYUKLILVYORSK-HBMCJLEFSA-N | [SMILES]
C(=O)(C1=CC=CC=C1)C[C@H]1CCC[C@@H](C[C@H](O)C2=CC=CC=C2)N1C |
| Hazard Information | Back Directory | [Description]
Lobeline is mainly present in Lobelia chinensis, Lobelia inflata, Campanula
medium, Lobelia hassleri, and Lobelia nicotianaefolia . Lobelia inflata has also
been considered as an Indian tobacco and has been used for the treatment of respiratory diseases for a long history. It was also a treatment of asthma by American
Aborigines. In the United States during the nineteenth century, doctors use Lobelia
inflata as a vomiting agent, to remove the poison from the body. It is also called
“vomit grass.” Now Lobelia inflata is still used to clear throat, bronchial, lung, and
other respiratory mucus . | [Chemical Properties]
Crystalline solid; melts at 130°C (266°F);slightly soluble in water, dissolves readily inhot alcohol, ether, chloroform, and benzene. | [Physical properties]
Appearance: White crystalline or granular powder, odorless, and bitter. Solubility:
Soluble in ethanol or chloroform, slightly soluble in water. Melting point:
130–131?°C. | [History]
In the 1930s, the chemical synthesis process of lobeline was completed, and its
artificial synthesis was realized, which was found in Lobelia inflata from the North
American Campanulaceae. It was commonly used as a lobeline hydrochloride.
Because of its structure similar to nicotine, it was initially used for the treatment of
respiratory diseases. Further study found that lobeline can selectively excite the
carotid sinus peripheral chemoreceptors, then reflect the excitement of the medullary breathing center, and enhance respiratory function. Therefore, it is widely used
as a respiratory stimulant .
Although lobeline showed similar biological activity with nicotine, its potency is
just only 1/5~1/20 of nicotine. Hence, lobeline was used as a substitute for nicotine in many smoking cessation products. However, in 1993, the Food and Drug
Administration (FDA) banned the sale of smoking cessation products containing
lobeline because it was ineffective in helping people quit smoking. However, the
research of lobeline in drug addiction still continues. | [Uses]
Lobeline is the principal lobelia alkaloid.It occurs in the seeds and herb of Indiantobacco (Lobelia inflata and Lobeliaceae). Itis used as a respiratory stimulant. Its sulfatesalt is used in antismoking tablets. | [Definition]
ChEBI: An optically active piperidine alkaloid having a 2-oxo-2-phenylethyl substituent at the 2-position and a 2-hydroxy-2-phenylethyl group at the 6-position. | [Indications]
Lobeline was recorded in chemical drug and preparations as lobeline hydrochloride,
which is prepared as injection for the treatment of central respiratory inhibition
induced by a variety of reasons. In addition, it is also used for the treatment of neonatal stasis, carbon monoxide, opioid poisoning, and so on. | [Health Hazard]
The structure of lobeline is different fromthose of nicotine and anabasine. It does nothave a pyridine ring, similar to the lattertwo alkaloids. However, its pharmacologicaction is similar to but less potent than thatof nicotine. Like anabasine, it is a respiratorystimulant. The toxic symptoms includeincreased salivation, nausea, vomiting, diarrhea,and respiratory distress. | [Pharmacology]
The pharmacological effects of lobeline are extensive, mainly manifested as
nicotine-like effect. On the one hand, lobeline can selectively excite the carotid
sinus and aortic body chemoreceptors and induce reflective excitement of the respiratory center and vagus center. Lobeline showed better excitatory effect for the
respiratory inhibition caused by morphine. There is bronchiectasis effect directly
when lobeline is inhaled, hence, against pilocarpine and acetylcholine-induced tracheal contraction. When the dose increased, lobeline can directly stimulate the
respiratory center and excite vagal center (reducing heart rate) and vomiting center
in medulla oblongata. In addition, lobeline showed dual role in the regulation of
ganglion, as manifested excitement and inhibition in chronological order. Lobeline
has a zebra-like effect on the striated muscle. In addition, it also has a certain anticancer effect, as manifested by significantly inhibiting the uptake of oxygen in
mouse ascites cancer cells . | [Clinical Use]
It is mainly used in the treatment of (1) neonatal asphyxia, (2) suffocation caused by
carbon monoxide, (3) poisoning induced by inhalation of anesthetics and other central inhibitors (such as opioids and barbiturates), and (4) respiratory failure caused
by pneumonia, diphtheria, and other infectious diseases. | [Side effects]
Lobeline also showed side
effects, such as nausea, vomiting, cough, headache, palpitations, and other adverse
reactions. | [Synthesis]
Lobeline, 1-methyl-2-(|?-hydroxy-|?-phenylethyl)-6-phenacylpiperidine (13.1.33),
is the primary alkaloid of leaves from Lobelia inflata. It is synthesized by condensation of 2,6-
dimethylpyridine with two moles of benzaldehyde, giving |á,|á??-distyrylpyridine (13.1.28)
[37¨C39]. Exhaustive bromination of this product and subsequent dehydrobromination of the
resulting tetrabromo derivative (13.1.29) leads to the formation of |á,|á??-diphenylethinylpyri�dine (13.1.30). Hydration of the triple bonds of the product (13.1.30) gives |á,|á??-
diphenacylpyridine (13.1.31). Reacting this with methyl p-toluenesulfonate gives
|á,|á??-diphenacylpyridinium N-methyl-p-toluenesulfonate (13.1.32), which is carefully
reduced by hydrogen into the desired lobeline (13.1.33) using simultaneously palladium and
platinum catalysts. The product is a racemic mixture from which the levorotatory isomer can
be isolated if necessary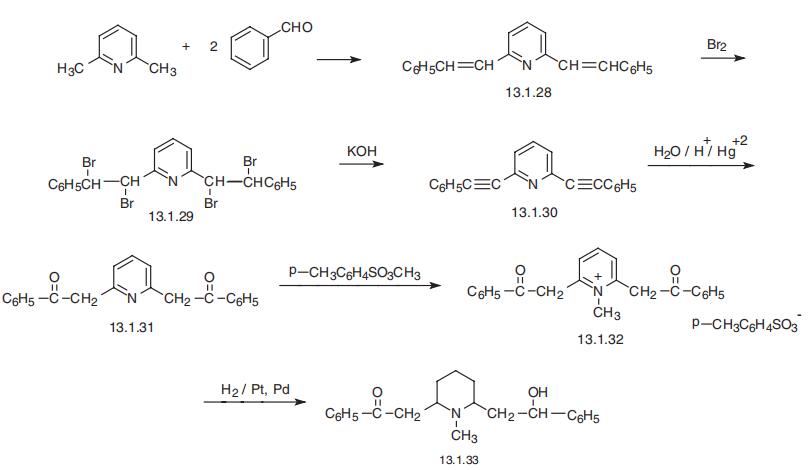
|
|
| Company Name: |
BOYAN PHARMA Gold
|
| Tel: |
18566078841 18566078841 |
| Website: |
boyanpharma.com/index.php?c=category&id=9 |
|