| Identification | Back Directory | [Name]
Dapagliflozin ((2S)-1,2-propanediol, hydrate) | [CAS]
960404-48-2 | [Synonyms]
CS-1307
Forxiga
Dapaglilozin
Enzymes Pepsase
Dapagliflozin Hydrate
Dapagliflozin Impurity 67
Dag column net a water glycol
BMS 512148 (2S)-1,2-propanediol
Dapagliflozin propanediol hydrate
Dapagliflozin Monohydrate In-House
Dapragliflozin Propanediol Monohydrate
BMS-512148 (2S)-1,2-propanediol, hydrate
Dapagliflozin propylene glycolate hydrate
Dapagliflozin (S)-Propylene Glycol Hydrate
Dapagliflozin ((2S)-1,2-propanediol, hydrate)
Dapagliflozin (2S)-1,2-Propanediol Monohydrate
Dapagliflozin,(2S)-1,2-propanediol, hydrate (1:1:1)
(1S)-1,5-Anhydro-1-C-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl
Dapagliflozin (API)
Dapagliflozin ((2S)-1,2-propanediol, hydrate)###461432-26-8###Dapagliflozin
(2S)-propane-1,2-diol (2S,3R,4R,5S,6R)-2-{4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl}-6-(hydroxymethyl)oxane-3,4,5-triol hydrate
(1S)-1,5-Anhydro-1-C-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-D-glucitol, compounded with (2S)-1,2-propanediol, hydrate (1:1:1)
(1S)-1,5-Anhydro-1-C-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-D-glucitol compound with (2S)-1,2-Propanediol (1:1), Monohydrate (9CI)
(2S,3R,4R,5S,6R)-2-(4-CHLORO-3-(4-ETHOXYBENZYL)PHENYL)-6-(HYDROXYMETHYL)TETRAHYDRO-2H-PYRAN-3,4,5-TRIOL COMPOUND WITH (S)-PROPANE-1,2-DIOL (1:1) HYDRATE | [EINECS(EC#)]
811-335-4 | [Molecular Formula]
C21H25ClO6.C3H8O2.H2O | [MDL Number]
MFCD28167768 | [MOL File]
960404-48-2.mol | [Molecular Weight]
502.982 |
| Chemical Properties | Back Directory | [Melting point ]
74 - 78°C | [storage temp. ]
-20°C Freezer | [solubility ]
Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) | [form ]
Solid | [color ]
White to Off-White | [Optical Rotation]
[α]/D +17 to +25, c =1.0 in chloroform-d | [InChIKey]
VYOCLVRXZCRBML-XDRSZCNYNA-N | [SMILES]
[C@H](O)(C)CO.O[C@@H]1[C@H]([C@H](O)[C@@H](CO)O[C@H]1C1C=CC(Cl)=C(CC2C=CC(OCC)=CC=2)C=1)O |&1:0,6,7,8,10,14,r| |
| Hazard Information | Back Directory | [Description]
Dapagliflozin propanediol monohydrate(960404-48-2) is the first approved sodium-glucose cotransporter protein 2 (SGLT2) inhibitor. It is indicated for the treatment of type 2 diabetes. When combined with diet and exercise in adults, dagliflozin helps improve glycaemic control by inhibiting glucose reabsorption in the proximal tubules of renal units and causing glycosuria. Dagliflozin can be given alone or in combination with insulin or other oral hypoglycaemic agents as adjunctive therapy.
Dagliflozin was originally approved by the FDA on 8 January 2014 for use in combination with diet and exercise to improve glycemic control in adults with type 2 diabetes. It was later approved in April 2021 to reduce the risk of declining kidney function, kidney failure, cardiovascular death and hospitalisation for heart failure in adults with chronic kidney disease.
| [Uses]
Dapagliflozin Propanediol Hydrate is an SGLT2 inhibitor, which can be used for the treatment of diabetes. | [Definition]
ChEBI: A hydrate that consists of dapagliflozin compounded with (S)-propylene glycol and hydrate in a (1:1:1) ratio. Used to improve glycemic control, along with diet and exercise, in adults with type 2 diabetes. | [Clinical Use]
The most likely process-scale synthesis has been described in a literature publication and patent, and
this is summarized in the scheme below. The synthesis began with global silylation glucolactone 63
to form tetrasiloxide 64. In parallel, commercial 5-bromo-2-chlorobenzoyl acid (65) was converted to
the corresponding acid chloride with oxalyl chloride. Subsequently, this acid chloride was subjected to
Friedel-Crafts acylation with ethyl phenyl ether (“phenetole”) in the presence of aluminum trichloride at
low temperature to give benzophenone 66 in 91% yield. Next, the carbonyl functionality within 66 was
removed upon treatment with triethylsilane and boron trifluoride-etherate, producing 5-bromo-2-chloro-
4'-ethoxydiphenylmethane 67 in 75% yield as the aglycon partner. Aryl bromide 67 was subjected to lithium halogen exchange conditions and subsequent exposure to lactone 64, which gave a mixture of
lactols which were then immediately subjected to methanesulfonic acid to provide glucol 68 in 85%
yield. The anomeric methoxy group of 68 was reduced with triethylsilane and boron trifluoride-etherate
followed by peracetylation to deliver α-C-glycoside tetra-acetate 69 in 55% (two steps) after
recrystalliaztion in ethanol. Hydrolysis of polyacetate 69 with lithium hydroxide gave dapagliflozin in
quantitative yield, and upon treatment with propanediol in water, dapagliflozin propanediol hydrate (X)
was produced. | [Synthesis]
Dapagliflozin propanediol hydrate, an orally active sodium glucose cotransporter type 2 (SGLT-2)
inhibitor, was developed by Bristol-Myers Squibb (BMS) and AstraZeneca for the once-daily treatment
of type 2 diabetes. As opposed to competitor SGLT-2 inhibitors, dapagliflozin was not associated with
renal toxicity or long-term deterioration of renal function in phase III clinical trials. The drug exhibits
excellent SGLT2 potency with more than 1200 fold selectivity over the SGLT1 enzyme.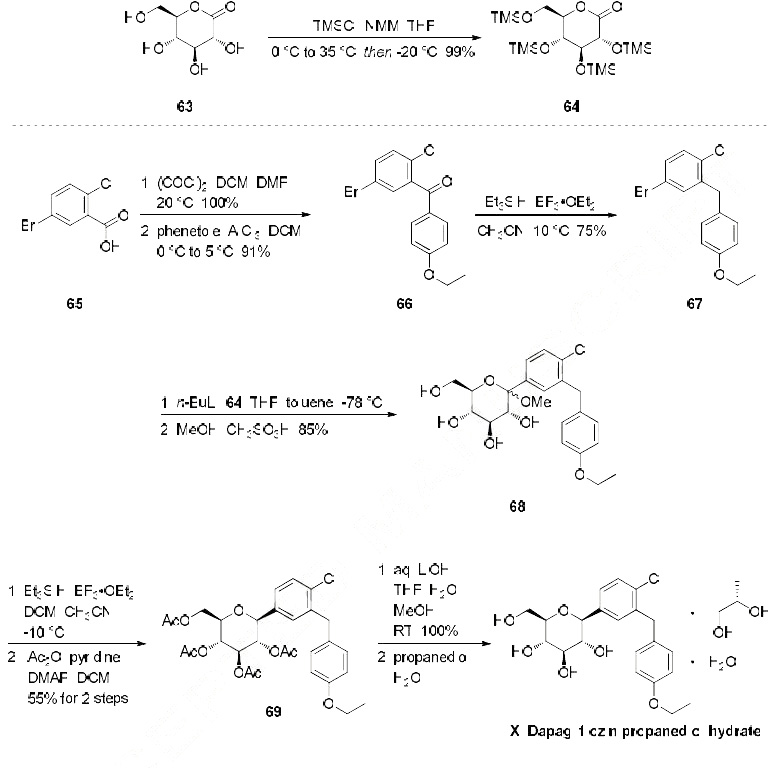
|
|
|