10034-20-5
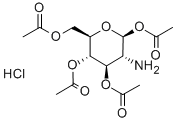 10034-20-5 结构式
10034-20-5 结构式
基本信息
2-氨基-2-脱氧-Β-D-吡喃葡萄糖 1,3,4,6-四乙酸酯盐酸盐
1,3,4,6-四-O-乙酰基-2-氨基-2-脱氧-Β-D-葡萄糖盐酸盐
1,3,4,6-Tetra-O-acetyl-a-D-glucosamineHCI
1,3,4,6-Tetra-O-acetyl-2-aMino-2-deoxy-beta-D-gluc
2-Amino-2-deoxy-β-D-glucopyranose 1,3,4,6-tetraacetate
1,3,4,6-Tetra-O-acetyl-beta-D-glucosamine hydrochloride
1,3,4,6-Tetra-O-acetyl-2-amino--D-glucopyranose, Hydrochloride
1,3,4,6-Tetra-o-acetyl-2-aMino-2-deoxy-beta-d-glucopyranose, HCl
2-Amino-2-deoxy-1,3,4,6-tetra-O-acetyl--D-glucopyranose Hydrochloride
1,3,4,6-Tetra-O-acetyl-2-aMino-2-deoxy-b-D-glucopyranose Hydrochloride
b-D-Glucopyranose, 2-aMino-2-deoxy-, 1,3,4,6-tetraacetate, hydrochloride
物理化学性质
制备方法
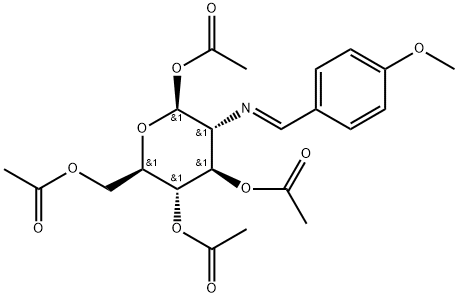
7597-81-1

5432-46-2
以(2S,3R,4R,5S,6R)-6-(乙酰氧基甲基)-3-(((E)-4-甲氧基亚苄基)氨基)四氢-2H-吡喃-2,4,5-三乙酸三酯(4.0 g, 8.6 mmol)为原料,将其溶解于预热的丙酮(36 mL)中。随后,向溶液中缓慢加入5 M HCl(2 mL),观察到沉淀生成。将反应混合物冷却至室温后,加入乙醚(36 mL)并持续搅拌2小时。通过过滤收集沉淀,用乙醚洗涤,并在减压下干燥,得到1,3,4,6-四-O-乙酰基-2-氨基-2-去氧-D-吡喃葡萄糖盐酸盐(2.9 g, 79%)为白色固体。产物的熔点为205°C(文献值:231-233°C,点燃[49],熔点235°C);比旋光度[α]20D +36.6(c 1.0, MeOH),文献值[49] +32(H2O);红外光谱(IR)νmax 2939 cm?1(宽峰,NH3Cl),1751 cm?1(C=O);1H-NMR(DMSO-d6, 300 MHz)δ 1.95(6H, s, CH3CO),2.00(3H, s, CH3CO),2.14(3H, s, CH3CO),3.52(1H, t, J = 9.7 Hz, H-2),3.98(2H, m, H-6, H-5),4.15(1H, dd, J = 12.3, 4.1 Hz, H-6),4.87(1H, t, J = 9.8 Hz, H-4),5.28(1H, t, J = 9.8 Hz, H-3),5.84(1H, d, J = 8.8 Hz, H-1),8.26(3H, s, NH3Cl)。
参考文献:
[1] Journal of Organic Chemistry, 1999, vol. 64, # 16, p. 5926 - 5929
[2] Journal of the American Chemical Society, 2018, vol. 140, # 8, p. 3120 - 3127
[3] Carbohydrate Research, 2010, vol. 345, # 11, p. 1541 - 1547
[4] Advanced Synthesis and Catalysis, 2010, vol. 352, # 14-15, p. 2657 - 2662
[5] RSC Advances, 2014, vol. 4, # 12, p. 6239 - 6245
