1020172-07-9
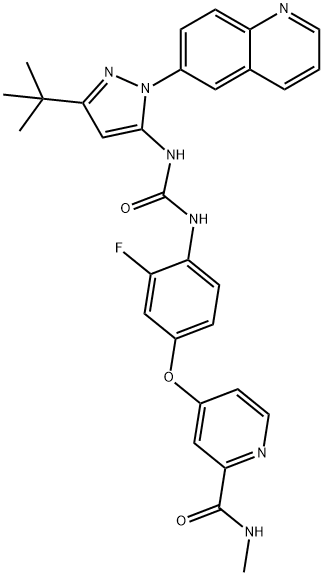 1020172-07-9 结构式
1020172-07-9 结构式
基本信息
N-[3-叔丁基-1-(喹啉-6-基)-1H-吡唑-5-基]-N'-[2-氟-4-[(2-(甲基氨基甲酰基)吡啶-4-基)氧]苯基]脲
Rebastinib
DCC2036
DCC 2036
Rebastinib Tosylate
Rebastinib(DCC-2036)
DCC-2036 (Rebastinib)
DCC2036
DCC-2036
DCC 2036
REBASTINIB.
4-(4-(3-(3-(tert-Butyl)-1-(quinolin-6-yl)-1H-pyrazol-5-yl)ureido)-3-fluorophenoxy)-N-methylpic
N-[3-tert-Butyl-1-(quinolin-6-yl)-1H-pyrazol-5-yl]-N'-[2-fluoro-4-[(2-(MethylcarbaMoyl)pyridin-4-yl)
4-(4-(3-(3-(tert-Butyl)-1-(quinolin-6-yl)-1H-pyrazol-5-yl)ureido)-3-fluorophenoxy)-N-methylpicolinamide
物理化学性质
制备方法
757251-39-1
![Carbamic acid, N-[3-(1,1-dimethylethyl)-1-(6-quinolinyl)-1H-pyrazol-5-yl]-, 2,2,2-trichloroethyl ester](/CAS/20210305/GIF/1011464-72-4.gif)
1011464-72-4
![N-[3-叔丁基-1-(喹啉-6-基)-1H-吡唑-5-基]-N'-[2-氟-4-[(2-(甲基氨基甲酰基)吡啶-4-基)氧]苯基]脲](/CAS/GIF/1020172-07-9.gif)
1020172-07-9
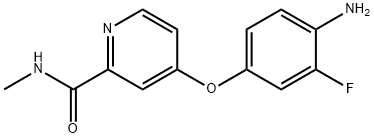
实施例1:将4-(4-氨基-3-氟苯氧基)-N-甲基吡啶-2-甲酰胺(7.0g,15.8mmol)、化合物(CAS:1011464-72-4,4.14g,15.8mmol)和N,N-二异丙基乙胺(DIEA,4.5g,34.9mmol)溶于二甲基亚砜(DMSO,70ml)中。将反应混合物置于70℃的油浴中加热反应8小时。反应完成后,将混合物倒入水(500ml)中,搅拌过夜,过滤收集固体。粗产物通过甲苯和丙酮连续结晶纯化,得到1-(3-叔丁基-1-(喹啉-6-基)-1H-吡唑-5-基)-3-(2-氟-4-(2-(甲基氨基甲酰基)吡啶-4-基氧基)苯基)脲,为白色结晶固体(4.06g,收率46%)。产物经1H NMR(DMSO-d6)和质谱(ESI)表征:1H NMR δ 8.90(m, 2H), 8.79(m, 1H), 8.52(m, 2H), 8.2(m, 3H), 7.96(dd, J = 9,2Hz, 1H), 7.63(dd, J = 8,4 Hz, 1H), 7.40(br s, 1H), 7.30(dd, J = 3,12 Hz, 1H), 7.17(m, 1H), 7.05(d, J = 9 Hz, 1H), 6.50(s, 1H), 2.80(d, J = 5Hz), 1.32(s, 9H); MS(ESI)m/z: 554(M + H+)。
参考文献:
[1] Patent: US2008/90856, 2008, A1. Location in patent: Page/Page column 49
[2] Patent: WO2013/36232, 2013, A2. Location in patent: Paragraph 00358
常见问题列表
| Target | Value |
|
u-Abl1 (native)
(Cell-free assay) | 0.75 nM |
|
Abl1 (H396P)
(Cell-free assay) | 1.4 nM |
|
p-Abl1 (native)
(Cell-free assay) | 2 nM |
|
FLT3
(Cell-free assay) | 2 nM |
|
p-Abl1 (T315I)
(Cell-free assay) | 4 nM |
DCC-2036有效抑制纯化的未磷酸化(u-ABL1 最新研究显示DCC-2036通过显著抑制CML细胞系(与非CML白血病细胞系相比),而选择性抑制BCR-ABL阳性细胞。
