1032528-06-5
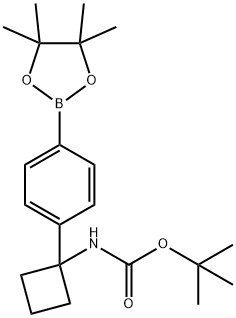 1032528-06-5 结构式
1032528-06-5 结构式
基本信息
(1-(4-(4,4,5,5-四甲基-1,3,2-二氧杂环戊硼烷-2-基)苯基)环丁基)氨基甲酸叔丁酯
tert-Butyl (1-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)
tert-butyl N-{1-[4-(tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]cyclobutyl}carbamate
tert-butyl 1-(4-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)phenyl)cyclobutylcarbaMate
Tert-butyl N-[1-[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]cyclobutyl]carbamate
[1-[4-(4,4,5,5-Tetramethyl-[1,3,2]dioxaborolan-2-yl)phenyl]cyclobutyl]carbamic acid tert-butyl ester
N-[1-[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]cyclobutyl]Carbamic acid 1,1-dimethylethyl ester
CarbaMic acid, N-[1-[4-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)phenyl]cyclobutyl]-, 1,1-diMethylethyl ester
物理化学性质
制备方法
![[1-(4-溴苯基)环丁基]氨基甲酸叔丁酯](/CAS/GIF/1032350-06-3.gif)
1032350-06-3

73183-34-3

1032528-06-5
步骤5:在500 mL脱气的四氢呋喃(THF)中,加入27.75 g(55 mmol)1-(4-溴苯基)环丁基氨基甲酸叔丁酯、23.76 g(93.6 mmol)联硼酸频那醇酯、25 g(255 mmol)乙酸钾和2.08 g(2.55 mmol)1,1'-双(二苯基膦基)二茂铁二氯化钯(II)。将反应混合物加热回流3小时,观察到反应混合物的颜色从深红色变为黑色。由于反应不完全,继续加热2小时。反应完成后,将反应混合物倒入400 mL水中,并用700 mL乙酸乙酯稀释。搅拌30分钟后,分离有机相,水相分别用400 mL和200 mL乙酸乙酯再萃取两次。合并有机萃取液,用200 mL盐水洗涤,并用硫酸钠干燥。蒸发溶剂后,通过Biotage色谱法纯化残余物,得到28.99 g(产率91.3%)1-(4-(4,4,5,5-四甲基-1,3,2-二氧硼戊环-2-基)苯基)环丁基氨基甲酸叔丁酯。1H NMR(400 MHz, DMSO-d6)δ: 7.51-7.67(m, 3H), 7.38(d, 2H), 2.22-2.42(m, 4H), 1.88-2.02(m, 1H), 1.63-1.80(m, 1H), 1.00-1.38(m, 21H)ppm。
参考文献:
[1] Patent: WO2013/104611, 2013, A1. Location in patent: Page/Page column 48
[2] Patent: WO2012/7416, 2012, A1. Location in patent: Page/Page column 75; 76
[3] Patent: US2014/5185, 2014, A1. Location in patent: Paragraph 0256-0258
[4] Patent: EP2868660, 2015, A1. Location in patent: Paragraph 0172
[5] Patent: WO2012/7345, 2012, A2. Location in patent: Page/Page column 243
