1080026-94-3
 1080026-94-3 结构式
1080026-94-3 结构式
基本信息
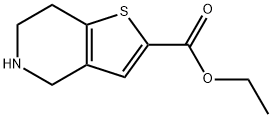
4,5,6,7-四氢噻吩[3,2-C]吡啶-2-甲酸乙酯
4,5,6,7-四氢噻吩并[3,2-C]吡啶-2-甲酸乙酯
4,5,6,7-四氢噻吩并[3,2-C]吡啶-2-羧酸乙酯
Ethyl 4,5,6,7-tetrahydrothieno[3,2-c]pyridine-2-carboxylate
4,5,6,7-Tetrahydrothieno[3,2-c]pyridine-2-carboxylic acid ethyl ester
Thieno[3,2-c]pyridine-2-carboxylic acid, 4,5,6,7-tetrahydro-, ethyl ester
制备方法
![5-BOC-4,5,6,7-四氢噻吩并[3,2-C]吡啶-2-羧酸乙酯](/CAS/GIF/623564-30-7.gif)
623564-30-7
![4,5,6,7-四氢噻吩[3,2-C]吡啶-2-甲酸乙酯](/CAS2/GIF/1080026-94-3.gif)
1080026-94-3
以5-叔丁氧羰基-4,5,6,7-四氢噻吩并[3,2-c]吡啶-2-羧酸乙酯为原料合成4,5,6,7-四氢噻吩并[3,2-c]吡啶-2-羧酸乙酯的一般步骤:在0℃下,向装有5-叔丁氧羰基-4,5,6,7-四氢噻吩并[3,2-c]吡啶-2-羧酸乙酯(1g,3.21mmol)的100mL圆底烧瓶中加入二氯甲烷(16mL),然后缓慢加入三氟乙酸(1.48mL,19.3mmol)。将反应混合物在室温下搅拌60分钟。反应完成后,向混合物中加入饱和碳酸氢钠水溶液(10mL)和二氯甲烷(10mL)。分离有机层后,水相用二氯甲烷(20mL)萃取两次。合并有机层,用饱和食盐水洗涤,无水硫酸镁干燥,减压浓缩,得到油状产物4,5,6,7-四氢噻吩并[3,2-c]吡啶-2-羧酸乙酯(0.547g,收率81%)。LCMS(FA)ES+:m/z 212。
参考文献:
[1] Patent: WO2010/151317, 2010, A1. Location in patent: Page/Page column 108
[2] Journal of Medicinal Chemistry, 2013, vol. 56, # 18, p. 7201 - 7211
[3] MedChemComm, 2016, vol. 7, # 5, p. 960 - 965
[4] Patent: WO2012/117421, 2012, A1. Location in patent: Page/Page column 40
[5] Patent: US2010/99664, 2010, A1. Location in patent: Page/Page column 32