109229-22-3
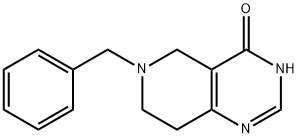 109229-22-3 结构式
109229-22-3 结构式
基本信息
6-苄基-5,6,7,8-四氢吡啶并[4,3-D]嘧啶-4(3H)-酮
6-苄基-5,6,7,8-四氢吡啶并[4,3-D]嘧啶-4(3H)-酮 6-BENZYL-5,6,7,8-TETRAHYDROPYRIDO[4,3-D]PYRIMIDIN-4(3H)-ONE
3-d]pyriMidin-4(3H)-one
6-benzyl-3H,4H,5H,6H,7H,8H-pyrido[4,3-d]pyriMidin-4-one
6-benzyl-1H,4H,5H,6H,7H,8H-pyrido[4,3-d]pyrimidin-4-one
6-Benzyl-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidin-4(3H)
Benzyl-5,6,7,8-tetrahydropyrido[4,3-d]pyriMidin-4(3H)-one
6-Benzyl-5,6,7,8-tetrahydro-1H-pyrido[4,3-d]pyriMidin-4-one
6-Benzyl-5,6,7,8-tetrahydro-3H-pyrido[4,3-d]pyrimidin-4-one
6-benzyl-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidin-4(3H)-one
6-(phenylmethyl)-1,5,7,8-tetrahydropyrido[4,3-d]pyrimidin-4-one
物理化学性质
制备方法
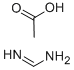
3473-63-0
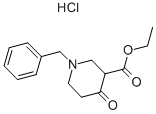
1454-53-1
![6-苄基-5,6,7,8四氢吡咯并[4,3-D]嘧啶-4(3H)-酮](/CAS/GIF/109229-22-3.gif)
109229-22-3
1. 在350 mL密封反应釜中,将1-苄基-4-氧代哌啶-3-羧酸乙酯盐酸盐(50.0 g,0.168 mol)、醋酸甲脒(16.2 g,0.201 mol)、4.37 M甲醇钠的甲醇溶液(190 mL)及甲醇(200 mL)混合。2. 将反应混合物加热至85℃,维持16小时。3. 反应完成后,冷却混合物并减压浓缩。4. 将浓缩残余物溶解于1 N NaOH溶液(150 mL)中,随后倒入冰水中。5. 缓慢加入冰醋酸调节混合物pH至7,析出棕褐色固体。6. 过滤收集固体,依次用水和冷乙醚洗涤。7. 高真空干燥后,得到6-苄基-5,6,7,8-四氢吡啶并[4,3-d]嘧啶-4(3H)-酮,为褐色固体(26.2 g,收率61.4%)。8. 质谱分析显示[M + H]+ = 242.2。9. 1H NMR (DMSO-d6) δ: 2.29 (t, J = 5.8 Hz, 2H), 2.61 (t, J = 5.8 Hz, 2H), 3.26 (s, 2H), 3.64 (s, 2H), 7.21-7.36 (m, 6H), 7.96 (s, 1H)。
参考文献:
[1] Patent: US2006/258689, 2006, A1. Location in patent: Page/Page column 22; 23; 27; 28
[2] Patent: US2008/275037, 2008, A1. Location in patent: Page/Page column 22
[3] Patent: WO2008/123963, 2008, A1. Location in patent: Page/Page column 44
[4] Patent: WO2011/103715, 2011, A1. Location in patent: Page/Page column 38
[5] Patent: WO2011/106276, 2011, A1. Location in patent: Page/Page column 38