116834-96-9
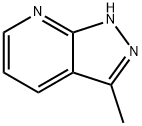 116834-96-9 结构式
116834-96-9 结构式
基本信息
3-甲基-1H-吡喃并-3,4-B]吡啶
3-甲基-(9CI)-1H-吡唑并[3,4-B]吡啶
3-METHYL-2H-PYRAZOLO[3,4-B]PYRIDINE
1H-Pyrazolo[3,4-b]pyridine,3-Methyl-
3-methyl-1H-pyrazolo[3,4-b]pyridine (en)
3-Methyl-1H-pyrazolo[3,4-b]pyridine ,97%
1H-Pyrazolo[3,4-b]pyridine,3-methyl-(9CI)
1H-Pyrazolo[3,4-b]pyridine,3-methyl-(9CI) ISO 9001:2015 REACH
物理化学性质
制备方法
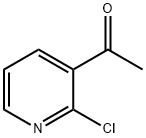
55676-21-6
![3-甲基-1H-吡唑并[3,4-B]吡啶](/CAS/GIF/116834-96-9.gif)
116834-96-9
步骤3:3-甲基-1H-吡唑并[3,4-b]吡啶的合成 将1-(2-氯吡啶-3-基)乙酮(22g,0.1415mol)与98%水合肼(113g,2.21mol)溶于乙醇(300mL)中,回流反应12小时。反应完成后,使用旋转蒸发器在减压下蒸馏除去约80%的乙醇。将残余物冷却至室温,过滤收集析出的固体,并用冷水洗涤。将所得产物于90℃下干燥至恒重,得到3-甲基-1H-吡唑并[3,4-b]吡啶(16g,收率85%),熔点为152-154℃。 1H NMR (CDCl3) δ: 8.62 (dd, J = 4.5, 1.4 Hz, 1H), 8.08 (dd, J = 8.0, 1.4 Hz, 1H), 7.15 (dd, J = 8.0, 4.5 Hz, 1H), 2.64 (s, 3H)。 MS (FAB) m/z: 133 (M++1)。
参考文献:
[1] European Journal of Medicinal Chemistry, 2012, vol. 57, p. 311 - 322
[2] Patent: US2006/47126, 2006, A1. Location in patent: Page/Page column 30
[3] Patent: WO2011/5759, 2011, A2. Location in patent: Page/Page column 77-78
[4] Canadian Journal of Chemistry, 1988, vol. 66, # 3, p. 420-428
[5] Patent: WO2011/84486, 2011, A1. Location in patent: Page/Page column 106; 111
