133081-25-1
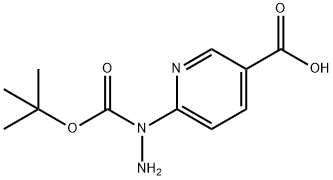 133081-25-1 结构式
133081-25-1 结构式
基本信息
6-(2-BOC-肼基)烟酸
6-叔丁氧羰肼基-3-吡啶甲酸
6-[2-(叔丁氧羰基)肼基]烟酸
6-(2-(叔丁氧基羰基)肼基)烟酸
6-[2-(叔丁氧羰基)肼基]烟酸供
4-HYDROXYETHANESULPHONICACID
6-Boc-hydrazinonicotinic acid
6-Boc-Hydrazynonicotinic acid
6-(2-Boc-hydrazino)nicotinic Acid
Butoxycarbonyl)hydrazinyl]nicotinic acid
6-(1-(tert-butoxycarbonyl)hydrazinyl)nicotinic acid
6-(2-(TERT-BUTOXYCARBONYL)HYDRAZINYL)NICOTINIC ACID
6-[2-(tert-Butoxycarbonyl)hydrazinyl]nicotinic acid USP/EP/BP
6-({[(tert-butoxy)carbonyl]aMino}aMino)pyridine-3-carboxylic acid
物理化学性质
制备方法
133081-24-0

24424-99-5
![6-[2-(叔丁氧羰基)肼基]烟酸](/CAS/GIF/133081-25-1.gif)
133081-25-1
向6-肼基吡啶-3-羧酸(0.346 g,1.82 mmol)和三乙胺(0.32 mL,2.36 mmol)的N,N-二甲基甲酰胺(DMF)溶液中加入二叔丁基二碳酸酯(0.40 g,1.82 mmol)。反应混合物在25℃下搅拌1小时后变得均匀,继续搅拌16小时。反应完成后,在减压下除去溶剂。将残余物用乙酸乙酯/甲醇(4:1,v/v)混合溶剂稀释,通过硅胶柱层析纯化,以乙酸乙酯为洗脱剂,得到6-[2-(叔丁氧羰基)肼基]烟酸(4b),为浅黄色固体(0.44 g,收率96%)。IR(ATR,cm-1):3353, 3227, 2975, 1719, 1593, 1472, 1372, 1256, 1014, 867, 794, 520。1H NMR(DMSO-d6,δ):12.584(s,1H),8.59(s,1H),7.97(d,J = 9 Hz,1H),6.54(d,J = 9 Hz,1H)。13C NMR(DMSO-d6,δ):166.6, 155.7, 150.5, 138.5, 116.7, 79.3, 28.1, 14.0。MS(ESI-):[M-H]- m/z 252.00。MS(ESI+):[M+H]+ m/z 254.07。HRMS(ESI+):[M+H]+ m/z 254.1145(计算值C11H16N3O4,254.1141)。
参考文献:
[1] Letters in Organic Chemistry, 2014, vol. 11, # 3, p. 208 - 214
[2] Bioorganic and Medicinal Chemistry Letters, 2009, vol. 19, # 16, p. 4764 - 4767
[3] Polish Journal of Chemistry, 2004, vol. 78, # 7, p. 951 - 959
[4] Journal of Labelled Compounds and Radiopharmaceuticals, 2018, vol. 61, # 2, p. 68 - 76
[5] Pharmazie, 2006, vol. 61, # 4, p. 269 - 273
