134218-81-8
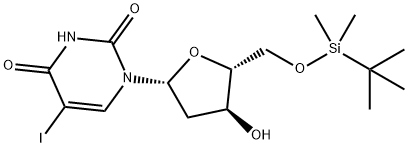 134218-81-8 结构式
134218-81-8 结构式
基本信息
5-碘-5'-TBDMS-2'-脱氧尿苷
2'-脱氧-5'-O-[(叔丁基)二甲基硅烷基]-5-碘尿苷
1-((2R,4S,5R)-5-(((叔丁基二甲基硅烷基)氧基)甲基)-4-羟基四氢呋喃-2-基)-5-碘嘧啶-2,4(1H,3H)-二酮
5'-O-TBS-5-iodo dU
5'-TBDMS-5-Iodo-2'-dU
5'-O-TBDMS-5-iodo-2'-deoxyuridine
2'-Deoxy-5'-O-TBDMS-5-Iodo-Uridine
2'-Deoxy-5'-O-TBDMS-5-Iodo-Uridine, >98%
5'-O-t-ButyldiMethylsilyl 2'-deoxy-5-iodo-uridine
5'-O-tert-Butyldimethylsilyl-2'-deoxy-5-iodouridine
5'-O-(TERT-BUTYLDIMETHYLSILYL)-5-IODO-2'-DEOXYURIDINE
5'-O-(TERT-BUTYLDIMETHYLSILYL)-5-IODO-2'-DEOXYURIDINE
物理化学性质
制备方法
54-42-2

18162-48-6
![2'-脱氧-5'-O-[(叔丁基)二甲基硅烷基]-5-碘尿苷](/CAS/GIF/134218-81-8.gif)
134218-81-8
一般步骤:向5-碘-2'-脱氧尿苷(5.0 g,14 mmol)的无水N,N-二甲基甲酰胺(DMF,70 mL)溶液中加入咪唑(1.09 g,16 mmol),随后在0℃下加入叔丁基二甲基氯硅烷(TBDMSCl,2.41 g,16 mmol)。将反应混合物置于冰浴中,搅拌过夜。反应完成后,用饱和NaHCO3水溶液淬灭,并用NaCl水溶液洗涤,随后以乙酸乙酯(EtOAc)萃取。有机层经无水硫酸镁(MgSO4)干燥后,减压浓缩除去溶剂。粗产物通过硅胶柱色谱纯化(洗脱剂:EtOAc/石油醚,3:7,v/v),得到目标产物1-((2R,4S,5R)-5-(((叔丁基二甲基硅烷基)氧基)甲基)-4-羟基四氢呋喃-2-基)-5-碘嘧啶-2,4(1H,3H)-二酮(5.9 g,收率90%),为无色固体。1H NMR (d6-DMSO) δ: 0.00 (s, 3H, CH3), 0.79 (s, 9H, tBu), 1.88-1.97 (m, 1H, H-2'), 2.00-2.05 (m, 1H, H-2'), 3.59-3.71 (m, 2H, H-5'), 3.75 (br s, 1H, H-4'), 4.06 (br s, 1H, H-3'), 5.18 (d, J = 4.0 Hz, 1H, OH), 5.98 (t, J = 5.9 Hz, 1H, H-1'), 7.89 (s, 1H, H-6), 11.62 (s, 1H, NH)。质谱(负电喷雾): 计算值C15H25IN2O5Si [M-H]- 468.06,实测值467。
参考文献:
[1] Patent: EP2607369, 2015, B1. Location in patent: Paragraph 0176
[2] Journal of Medicinal Chemistry, 2004, vol. 47, # 7, p. 1840 - 1846
[3] Patent: WO2017/58953, 2017, A1. Location in patent: Paragraph 0905; 0906
[4] European Journal of Medicinal Chemistry, 2015, vol. 101, p. 668 - 680
[5] European Journal of Medicinal Chemistry, 2016, vol. 120, p. 304 - 312
